Polyhydroxypropyl arginine and its derivative polyhydroxy saccharide compound as well as preparation method and application thereof
A technology of polyhydroxypropyl arginine and hydroxypropyl arginine, which is applied in the field of daily chemical products, can solve the problem of low adsorption performance and deposition efficiency of moisturizing agent and skin/hair surface, and it is difficult to form an intermolecular network composite film structure. , can not form a complex hydrogen bond structure and other problems, to achieve excellent moisturizing effect, the preparation method is simple and easy to operate, and the preparation method is simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: an aqueous solution of 1% trehalose and 0.5% dihydroxypropyl arginine. Wherein, the structure of dihydroxypropyl arginine is as shown in formula I, wherein X=H, Y=H; that is, the structure of dihydroxypropyl arginine is as shown in formula X:
[0053]
Embodiment 2
[0054] Example 2: 1% trehalose, 0.5% glycerin aqueous solution.
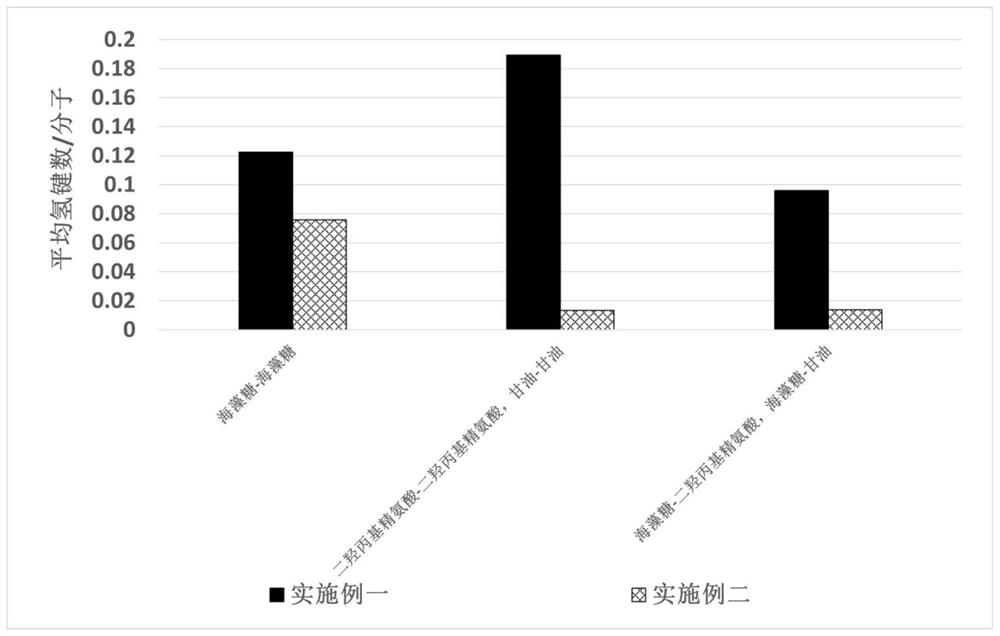
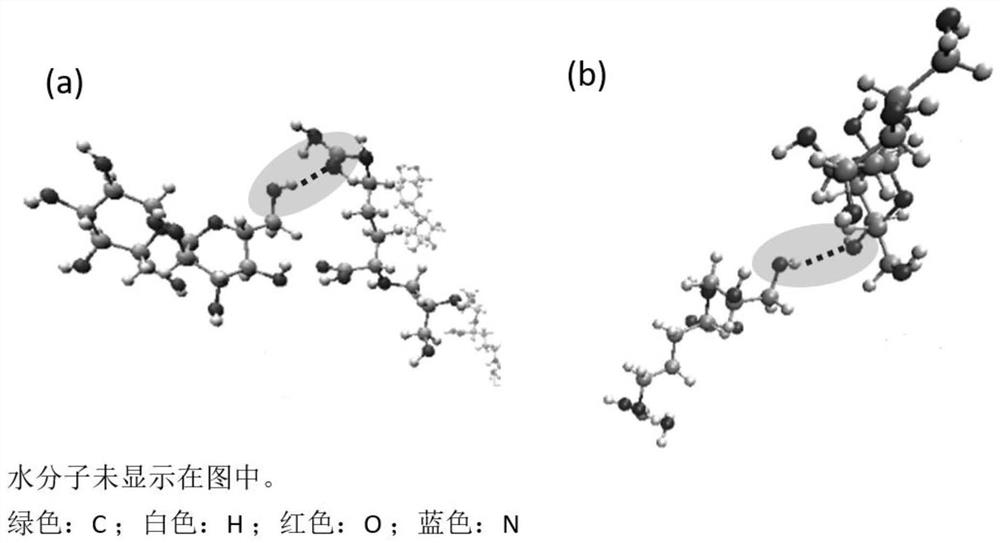
[0055] Calculated as figure 1 As shown, when the system is in balance, there is a strong hydrogen bond between the molecules of Example 1, especially the hydrogen bond between the dihydroxypropyl arginine molecule and the biologically active sugar trehalose molecule, which is far greater than that between the glycerol molecule and the trehalose molecule. Hydrogen bonding between trehalose. By observing the intermolecular hydrogen bond conformation after the system reaches thermodynamic equilibrium, such as figure 2 As shown, there are mainly two hydrogen bond conformations between the dihydroxypropyl arginine molecule and the trehalose molecule used in the present invention, OH-N hydrogen bond and OH-H hydrogen bond. It does not exist in the system of small molecule glycerol and trehalose. The above results verified the special intermolecular strong hydrogen bond interaction mechanism between dihydroxypropyl...
Embodiment 3
[0061] Example 3: 1% trehalose aqueous solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com