Synthesis of glycyrrhizic acid nanoparticles and combined treatment application of glycyrrhizic acid nanoparticles in novel coronavirus pneumonia
A technology of nanoparticles and glycyrrhizic acid, which is applied in the direction of antiviral agents, medical preparations containing active ingredients, anti-inflammatory agents, etc., can solve the problems of limited therapeutic potential, poor solubility, and low bioavailability, and achieve improved biophase Poor capacitance, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
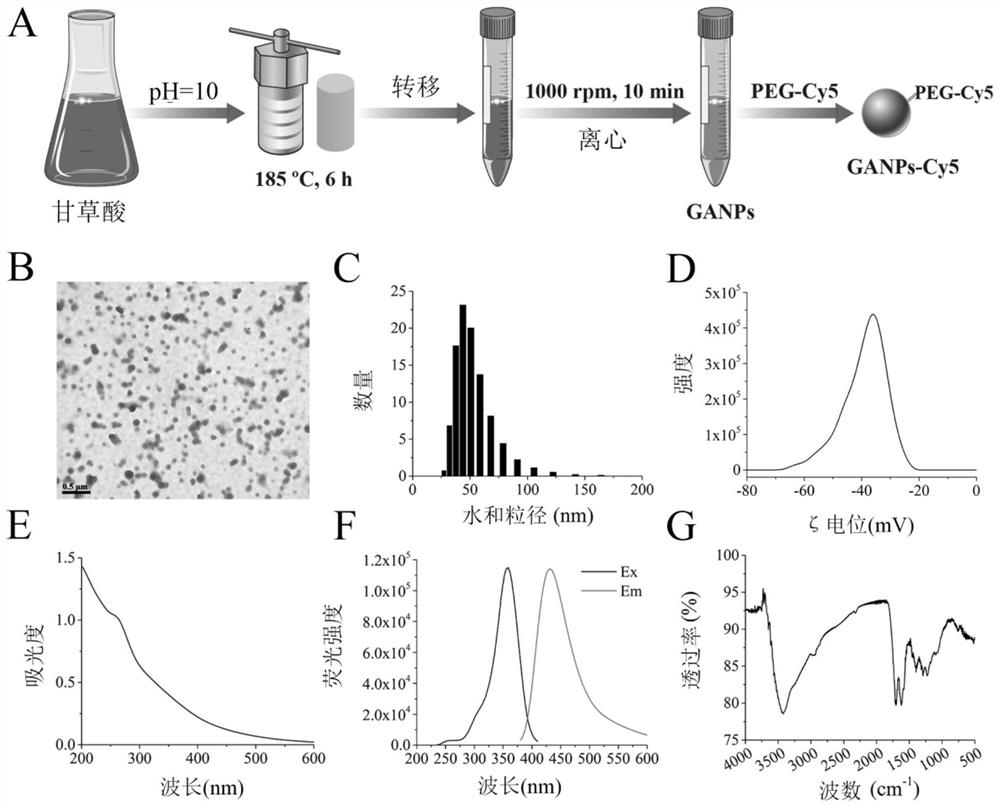
[0030] Example 1 Synthesis and Characterization of Glycyrrhizic Nanoparticles (GANPS)
[0031] When the glycyrrhizic nanoparticles were synthesized, glycyrrhic acid was used as a carbon source, and the pH was adjusted to 10.0 ± 0.5 using a saturated sodium hydroxide solution, followed by incubation at 180 ° C for 7 h.
[0032] The synthesis of glycyrrhizic nanoparticles mainly includes the following steps:
[0033] A, 0.10 g (0.12 mmol) glycyrrhizic acid dissolved in 10 ml deionized water;
[0034] B, then a drop of saturated a saturated sodium hydroxide solution was adjusted to 10.0 ± 0.5;
[0035] C, poured the mixture into a 25 mL of the polytetrafluoroethylene lining, incubation for 7 h at 180 ° C;
[0036] D. After nature is cooled to room temperature, 10 000 rpm is centrifuged for 10 min from the mixture from the mixture;
[0037] E, subsequently collecting the upper cleaner, and the small precipitate is further removed by filtration by 0.22 μm filter;
[0038] f, Finally, u...
Embodiment 2
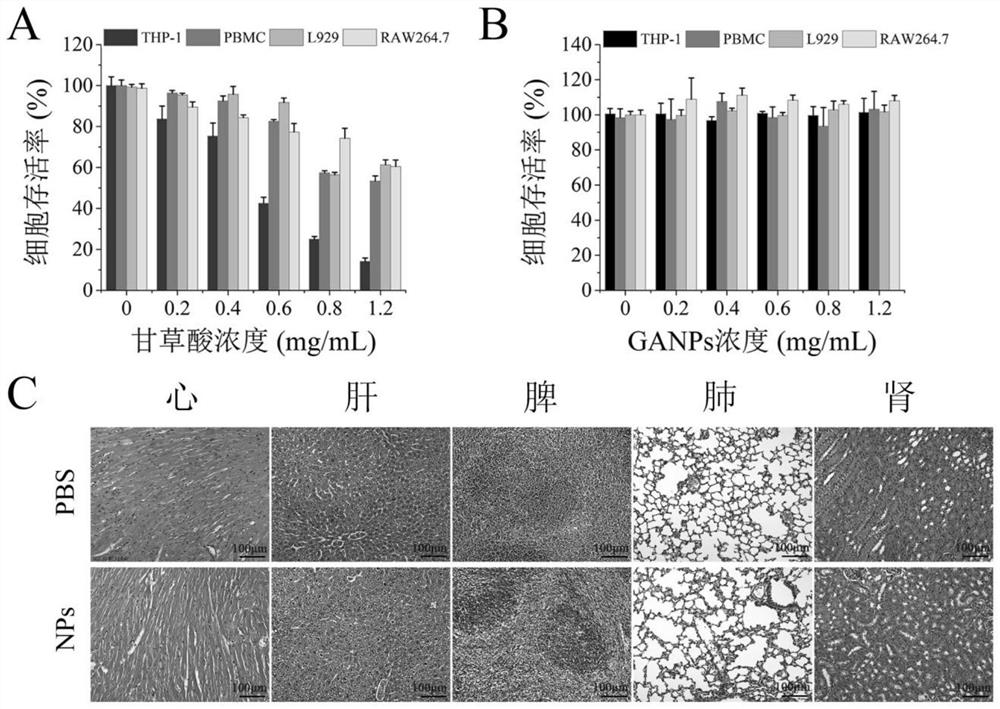
[0042] Example 2Ganps of biocompatibility assessment
[0043] Board, 96-well plate (generally in flat), the number of cells is 5000 tell / well, 100 ul medium per hole; formulated different material concentration gradient (0 mg / ml, 0.2 mg / ml, 0.4 mg / ml, 0.6 mg) / ml, 0.8 mg / ml, 1.2 mg / ml) (6 times each set of concentration gradients); each hole corresponds to 100 ul, one vertical row, one vertical row. (From left to right, low concentration to high concentration); 37 ° C overnight incubation 24h; configure CCK8 solution, more equipped with a hole (43 holes), 430 ul of CCK8 solution to add 4.000 ml of medium; discard the original cultures in the hole Base, each hole is added to 100 ul (first control group, and then the material concentration is low to high plus, longitudinally sampled), and the ultraviolet absorption peak was measured at 37 ° C, and the ultraviolet absorption peak was measured every 10 minutes. The experiment can be terminated when the control group OD is ...
Embodiment 3
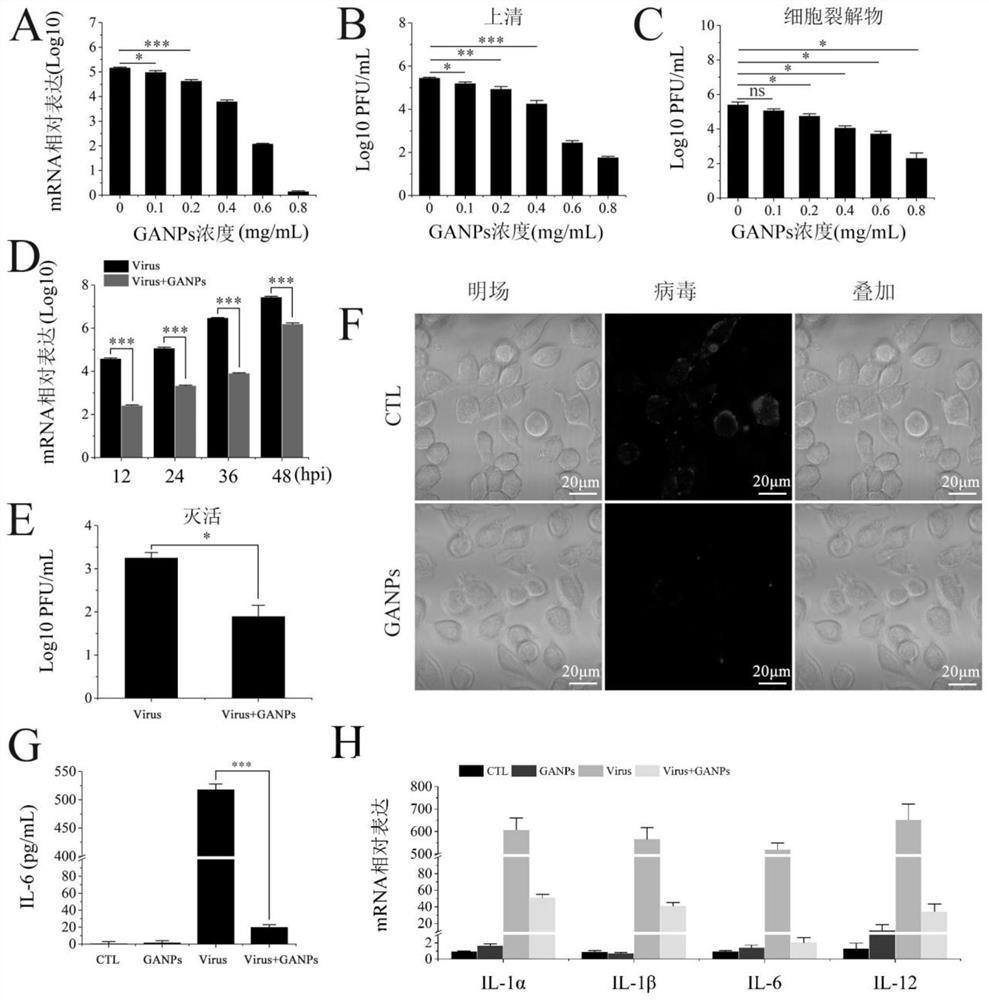
[0050] Example 3GANPS for in vitro treatment effects of SARS-COV-2 infected cells
[0051] Briefly, L929 cells were incubated for 2 hours in DMEM (containing 2% fetal bovine serum) and 0.40 mg / ml Ganps, and viruses were incubated at 4 ° C and 0.40 mg / ml Ganps for 1 hour. Then, the medium containing Ganps was removed, and the pretreated MOI was replaced by a virus 1. After incubating the incubator for 1 h, discard it, washed twice with DMEM, and then incubated with different doses of Ganps for 24 h. The inhibitory effect of Ganps on viral infection was evaluated with a mild spot method and RT-QPCR. The supernatant was collected at a different point in time, and then fresh medium was added to the cells, and the cells were stored at -80 ° C; after freezing, the sample was collected as a cell lysate. All collected samples are stored at -80 ° C and quantify the number of virals in the sample through air spots.
[0052] The number of viral particles was detected with a vacuum test, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com