Process for preparing triphenylchloromethane
A triphenylchloromethane, process technology, applied in the field of preparation of triphenylchloromethane, to achieve the effect of improving recycling, avoiding pollution problems, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
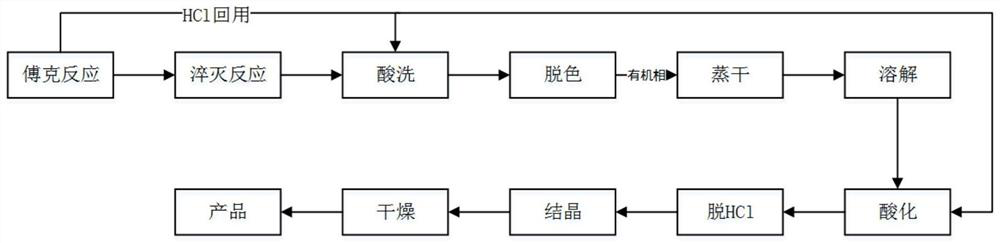
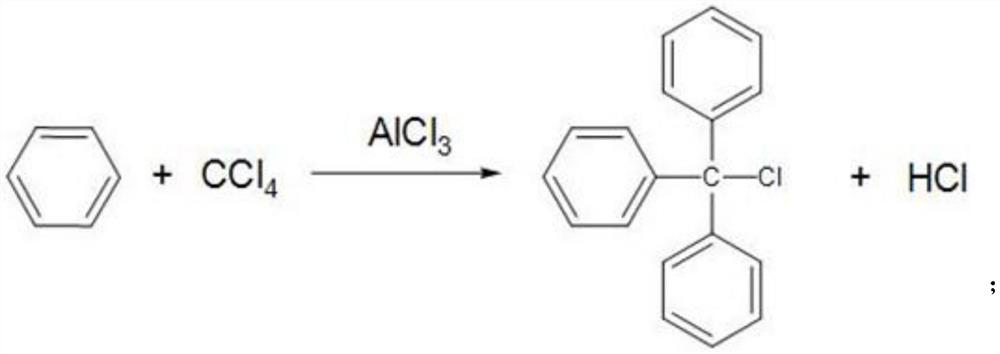
[0043] Put 130g of pure benzene and 25g of anhydrous aluminum trichloride into a 250mL four-neck flask, control the temperature of the water bath at 25°C, slowly add carbon tetrachloride dropwise for 4 hours; Continue to raise the temperature to 78°C, carry out the reflux reaction for 3 hours, then lower the temperature to 25°C, keep the temperature for 30 minutes, and obtain the Friedel-Crafts reaction solution for later use, and collect the HCl gas generated during the process;
[0044] Put 88g of pure benzene and 150g of 15% hydrochloric acid aqueous solution into a 500mL four-neck flask. The temperature of the water bath is controlled at 10°C. Add the Friedel-Crafts reaction solution dropwise to the reaction flask for 0.5h, and continue to stir for 10min after the dropwise addition is completed. , stop stirring, separate layers after standing for 30min, remove the water layer, and wash the oil phase twice with 150g of 15% hydrochloric acid aqueous solution;
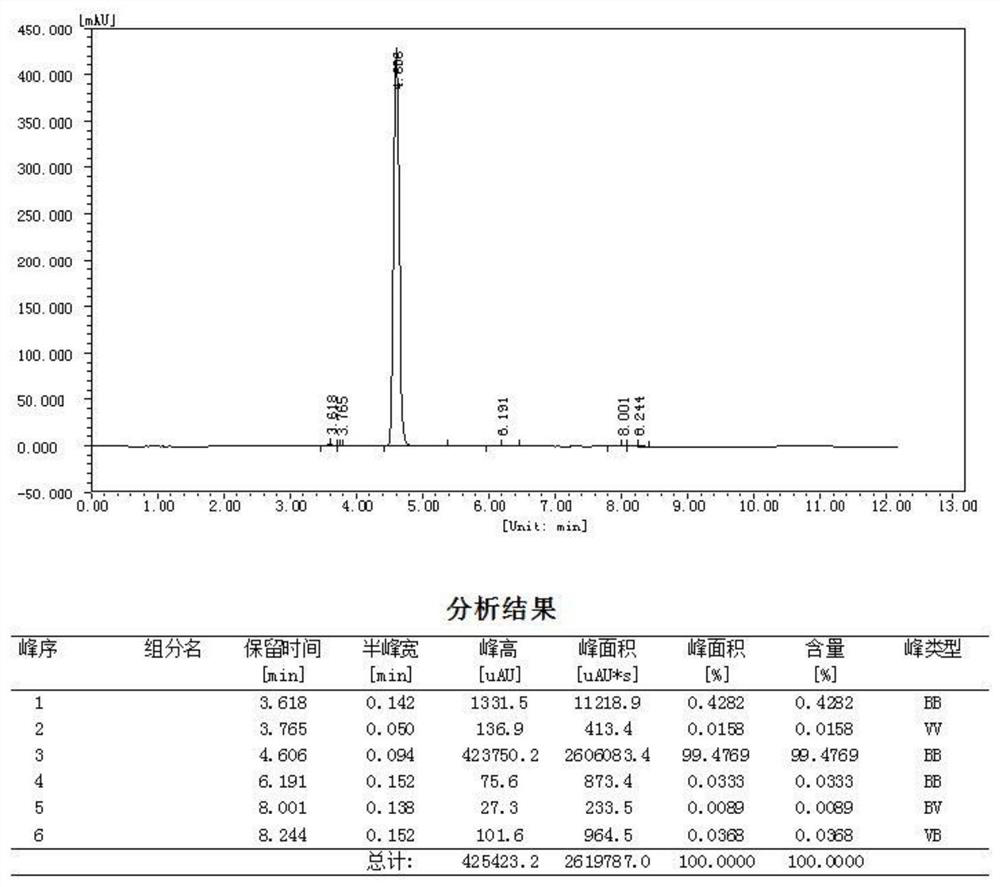
[0045] Collec...
Embodiment 2
[0049] Put 135g of pure benzene and 28g of anhydrous aluminum trichloride into a 250mL four-neck flask, control the temperature of the water bath at 30°C, slowly add carbon tetrachloride dropwise for 3.5h; after the addition of carbon tetrachloride, keep it warm for 2h Then continue to heat up to 70°C, carry out the reflux reaction for 3 hours, then lower the temperature to 25°C, keep it warm for 30 minutes, and obtain the Friedel-Crafts reaction solution for later use, and collect the HCl gas generated during the process;
[0050] Put 88g of pure benzene and 150g of 25% hydrochloric acid aqueous solution into a 500mL four-necked flask. The temperature of the water bath is controlled at 10°C. Add the Friedel-Crafts reaction solution dropwise to the reaction flask for 0.5h. After the dropwise addition, continue to stir for 10min. , stop stirring, separate layers after standing for 30min, remove the water layer, and wash the oil phase twice with 150g of 25% hydrochloric acid aque...
Embodiment 3
[0055] Put 265g of pure benzene and 60g of anhydrous aluminum trichloride into a 500mL four-neck flask, control the temperature of the water bath at 35°C, and slowly add carbon tetrachloride dropwise for 3.5h. After the carbon tetrachloride was added dropwise, keep warm for 2 hours and continue to heat up to 80°C. After reflux reaction for 3 hours, the temperature drops to 25°C. Keep warm for 30 minutes to get Friedel-Crafts reaction solution for later use; wherein, the HCl gas generated during the process is collected.
[0056] Put 160g of pure benzene and 350g of 20% hydrochloric acid aqueous solution into a 1000mL four-neck flask, control the temperature of the water bath at 10°C, add the spare material for Friedel-Crafts reaction to the reaction flask dropwise, the dropwise addition time is 0.5h, and continue to stir after the dropwise addition is completed After 10 minutes, stop stirring, let stand for 30 minutes, separate layers, remove the water layer, and wash the oil p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com