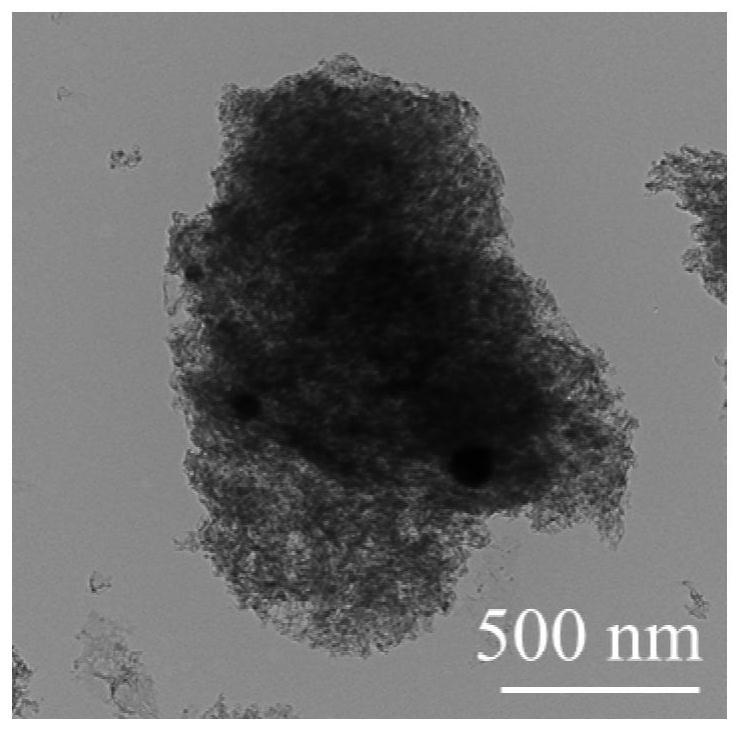
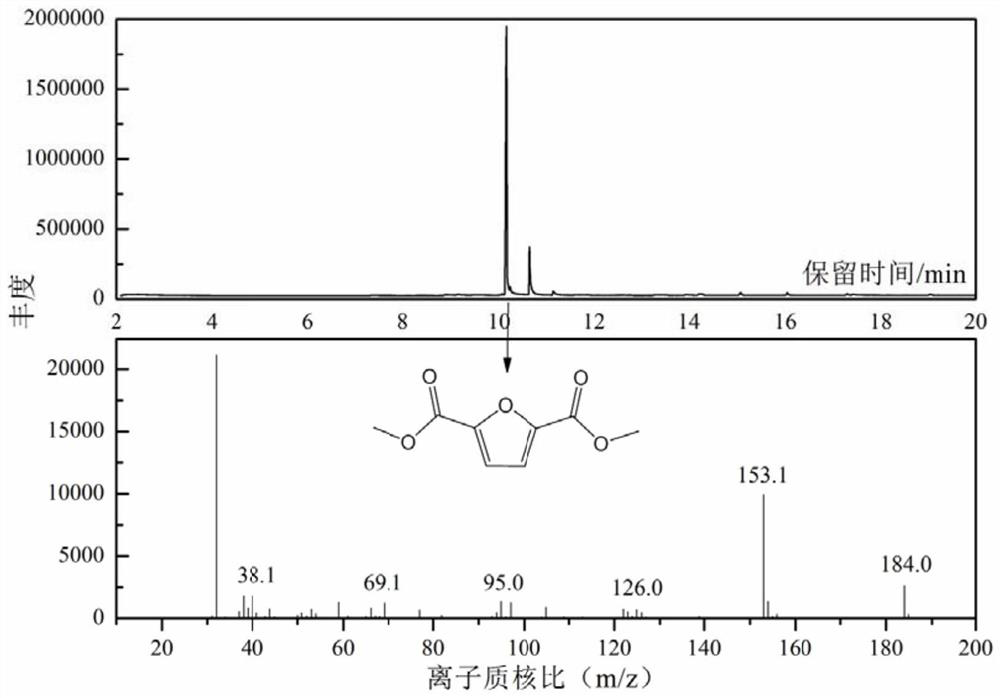
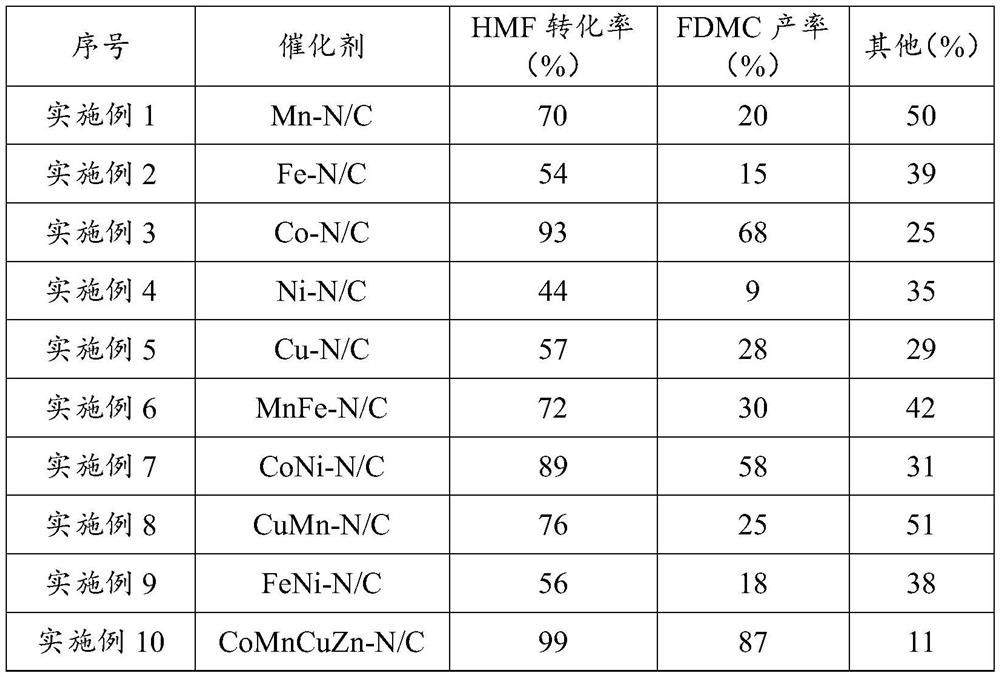
Method for preparing dimethyl furan-2,5-dicarboxylate
A technology of dimethyl furandicarboxylate and hydroxymethyl furfural, which is applied in chemical instruments and methods, catalyst activation/preparation, organic chemistry, etc., can solve the problem that the oxidation of 5-hydroxymethyl furfural is difficult and the metal resources are not suitable for extensive use. It can save the preparation cost, facilitate the activity, and avoid the agglomeration effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1. Preparation of non-noble metal catalyst Mn-N / C:
[0062] (1) Prepare non-precious metal salt impregnation solution: take pyridine and manganese acetate with a mass ratio of 3:2, add them to 30mL of methanol, and stir thoroughly for 1h;
[0063] (2) Add nitrogen-containing carrier urea to the non-precious metal salt impregnating solution configured in step (1), wherein the mass ratio of nitrogen-containing carrier urea to the manganese acetate added in step (1) is 10:2, fully impregnated;
[0064](3) Drying the non-precious metal salt impregnating solution impregnated with the nitrogen-containing carrier obtained in step (2) at 60° C., and loading the non-noble metal complex precursor;
[0065] (4) in N 2 Under the atmosphere, pyrolyze the non-noble metal-loaded complex precursor obtained in step (3). The pyrolysis condition is to raise the temperature to 800 °C at a rate of 10 °C / min, and keep it at this temperature for 2 hours to obtain a non-noble metal catalyst. ...
Embodiment 2
[0071] 1. Preparation of non-precious metal catalyst Fe-N / C:
[0072] (1) Prepare non-precious metal salt impregnation solution: take imidazole and ferric sulfate with a mass ratio of 3:4, add them to 40mL of methanol, and stir thoroughly for 1h;
[0073] (2) Add nitrogen-containing carrier melamine to the non-precious metal salt impregnating solution configured in step (1), wherein the mass ratio of nitrogen-containing carrier melamine to the ferric sulfate added in step (1) is 10:2, fully impregnated;
[0074] (3) Drying the non-precious metal salt impregnating solution impregnated with the nitrogen-containing carrier obtained in step (2) at 60° C., and loading the non-noble metal complex precursor;
[0075] (4) Under an Ar atmosphere, pyrolyze the non-noble metal-loaded complex precursor obtained in step (3). The temperature was kept for 2 hours to obtain the non-noble metal catalyst Fe-N / C.
[0076] 2. Oxidative esterification of 5-hydroxymethylfurfural:
[0077] (1) Pu...
Embodiment 3
[0081] 1. Preparation of non-precious metal catalyst Co-N / C:
[0082] (1) Prepare non-precious metal salt impregnation solution: take 1,10-phenanthroline and cobalt nitrate with a mass ratio of 1:1, add them to 50mL of methanol, and stir thoroughly for 1h;
[0083] (2) Nitrogen-containing carrier dicyandiamide is added to the non-precious metal salt impregnating solution configured in step (1), wherein the mass ratio of nitrogen-containing carrier dicyandiamide to the cobalt nitrate added in step (1) is 10: 2. Full impregnation;
[0084] (3) Drying the non-precious metal salt impregnating solution impregnated with the nitrogen-containing carrier obtained in step (2) at 60° C., and loading the non-noble metal complex precursor;
[0085] (4) in N 2 Under the atmosphere, pyrolyze the non-noble metal-loaded complex precursor obtained in step (3). The pyrolysis condition is to raise the temperature to 800° C. at a rate of 10° C. / min due to 25° C., and keep it at this temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com