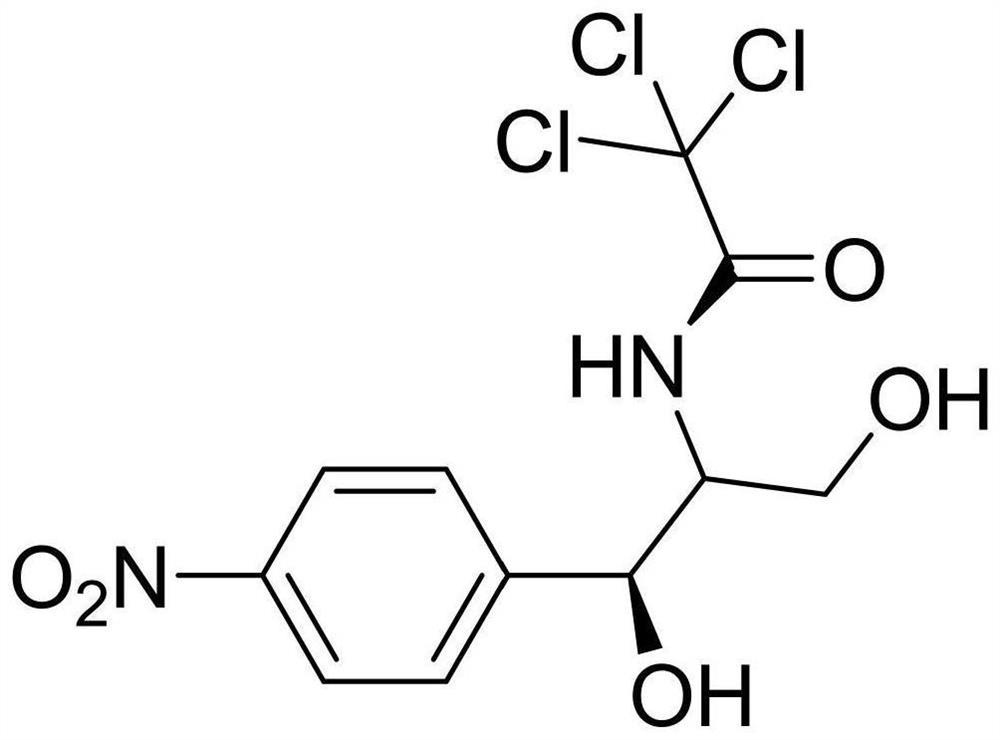
Preparation method of chloramphenicol impurity compound I
A compound, the technology of chloramphenicol, which is applied in the field of preparation of chloramphenicol impurities, can solve the problems of low yield and purity, long reaction time, cost of production enterprises, etc., achieve high yield and purity, save manpower and material resources, and react short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
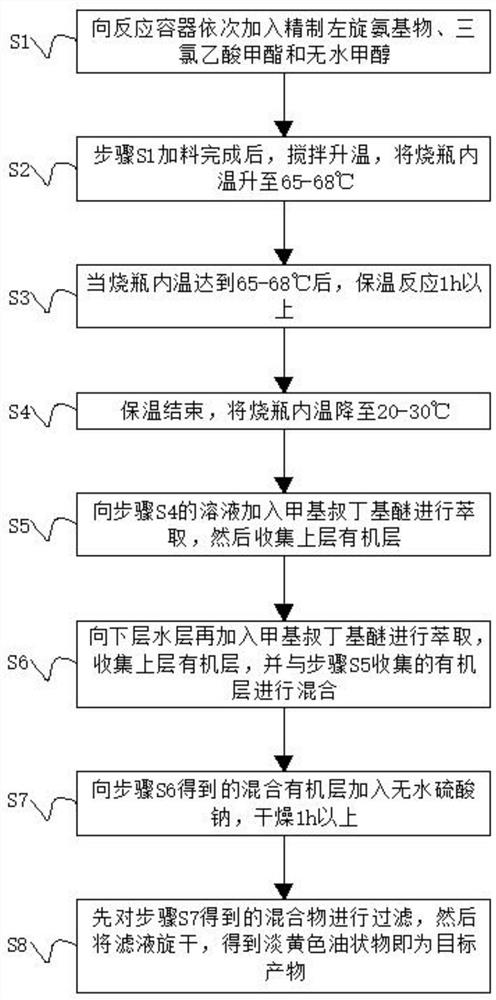
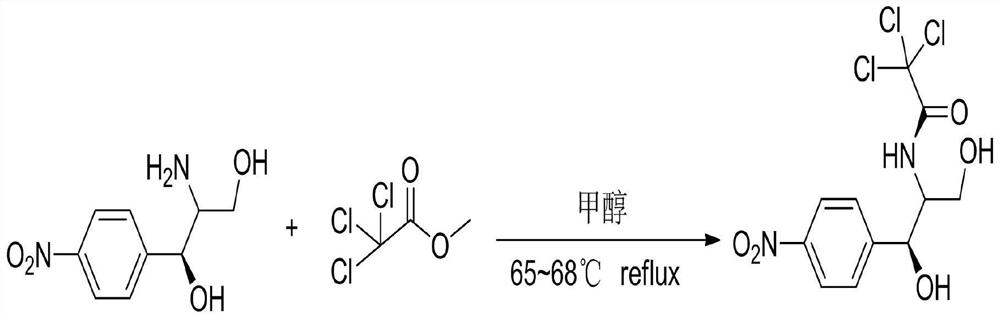
[0031] S1. Add 10.0 g of refined L-amino, methyl trichloroacetate and 10.0 g of anhydrous methanol to the three-necked flask in sequence;
[0032] S2. After the feeding in step S1 is completed, stir and heat up, and raise the temperature inside the flask to 67°C;
[0033] S3. When the inner temperature of the flask reaches 67°C, the heat preservation reaction time is 1.25h;
[0034] S4, the heat preservation is finished, and the inner temperature of the flask is lowered to 25° C.;
[0035] S5, adding 20ml of methyl tert-butyl ether to the solution in step S4 for extraction, and then collecting the upper organic layer;
[0036] S6, adding 20ml of methyl tert-butyl ether to the lower aqueous layer for extraction, collecting the upper organic layer, and mixing with the organic layer collected in step S5, repeating the above operation, and extracting 3 times with methyl tert-butyl ether;
[0037] S7, adding anhydrous sodium sulfate to the mixed organic layer obtained in step S6 ...
Embodiment 2
[0043] S1. Add 10.0 g of refined L-amino and 9.20 g of methyl trichloroacetate to the three-necked flask in sequence
[0044] and anhydrous methanol 10.0g;
[0045] S2, after the addition of step S1 is completed, stir and heat up, and the temperature in the flask is raised to T;
[0046] S3, when the internal temperature of the flask reaches T, the heat preservation reaction time is 1.25h;
[0047] S4, the heat preservation is finished, and the inner temperature of the flask is lowered to 25° C.;
[0048] S5, adding 20ml of methyl tert-butyl ether to the solution in step S4 for extraction, and then collecting the upper organic layer;
[0049] S6, adding 20ml of methyl tert-butyl ether to the lower aqueous layer for extraction, collecting the upper organic layer, and mixing with the organic layer collected in step S5, repeating the above operation, and extracting 3 times with methyl tert-butyl ether;
[0050] S7, adding anhydrous sodium sulfate to the mixed organic layer obt...
Embodiment 3
[0056] S1. Add 10.0 g of refined L-amino and 9.20 g of methyl trichloroacetate to the three-necked flask in sequence
[0057] and anhydrous methanol 10.0g;
[0058] S2. After the feeding in step S1 is completed, stir and heat up, and raise the temperature inside the flask to 67°C;
[0059] S3. When the internal temperature of the flask reaches 67°C, the heat preservation reaction time is t;
[0060] S4, the heat preservation is finished, and the inner temperature of the flask is lowered to 25° C.;
[0061] S5, adding 20ml of methyl tert-butyl ether to the solution in step S4 for extraction, and then collecting the upper organic layer;
[0062] S6, adding 20ml of methyl tert-butyl ether to the lower aqueous layer for extraction, collecting the upper organic layer, and mixing with the organic layer collected in step S5, repeating the above operation, and extracting 3 times with methyl tert-butyl ether;
[0063] S7, adding anhydrous sodium sulfate to the mixed organic layer ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com