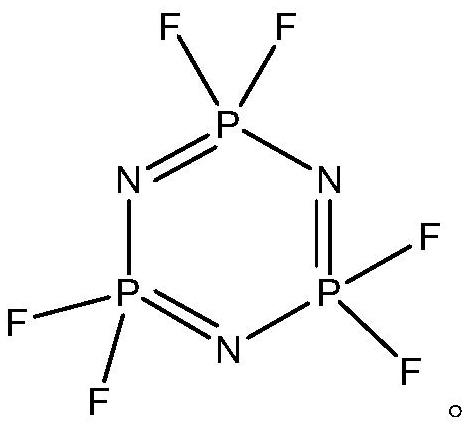
Preparation method of hexafluorocyclotriphosphazene
A technology of hexafluorocyclotriphosphazene and hexachlorocyclotriphosphazene, which is applied in the field of preparation of hexafluorocyclotriphosphazene, can solve the problems of unreachable product yield, low boiling point of cocatalyst, poor separation effect, etc., and achieve The effect of short preparation time, complete reaction and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: In a 2000mL three-neck flask equipped with an electric stirrer, a reflux condenser, and a thermometer, add 600g of n-hexane, cool down to 0°C, add 200g of hexachlorocyclotriphosphazene and 200g of potassium fluoride, and stir evenly. 10 g of ethylene glycol monomethyl ether and 10 g of ethylene glycol monobutyl ether were added dropwise, and the temperature was raised to 50° C. for reaction, and then the temperature was lowered to 20° C. and kept for 1 hour of reaction. There is no hexachlorocyclotriphosphazene residue in the reaction solution, and the reaction is complete. 128 g of hexafluorocyclotriphosphazene were obtained by rectification, with a molar yield of 90.1%.
Embodiment 2
[0020] Example 2: In a 2000mL three-necked flask equipped with an electric stirrer, a reflux condenser, and a thermometer, add 400g of n-hexane, cool down to -10°C, add 200g of hexachlorocyclotriphosphazene and 200g of potassium fluoride, and stir evenly , 16 g of ethylene glycol monomethyl ether and 24 g of ethylene glycol monobutyl ether were added dropwise, the temperature was raised to 52° C. for reaction, and then the temperature was lowered to 25° C. and kept for 2 hours for reaction. There is no hexachlorocyclotriphosphazene residue in the reaction solution, and the reaction is complete. 125 g of hexafluorocyclotriphosphazene was obtained by rectification, with a molar yield of 88.0%.
Embodiment 3
[0021] Example 3: In a 2000mL three-neck flask equipped with an electric stirrer, a reflux condenser, and a thermometer, add 500g of n-hexane, cool down to 20°C, add 200g of hexachlorocyclotriphosphazene and 200g of potassium fluoride, and stir evenly. 20 g of ethylene glycol monomethyl ether and 2 g of ethylene glycol monobutyl ether were added dropwise, and the temperature was raised to 30° C. for 2 hours to react. 50% of hexachlorocyclotriphosphazene remains in the reaction solution, and the reaction is not complete. Finally, rectification obtained 70 g of hexafluorocyclotriphosphazene with a molar yield of 49.3%. In this embodiment, the catalyst ratio is 1:0.1, although the reaction can be carried out, but the reaction is not complete, which is not preferred.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com