Preparation, renaturation and preservation method of VacA recombinant protein
A technology of recombinant protein and preservation method, which is applied in the field of preparation, renaturation and preservation of VacA recombinant protein, which can solve the problems of difficult purification, difficulty in interpreting and analyzing proteins, and heavy workload, and achieve the effect of reducing cell volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
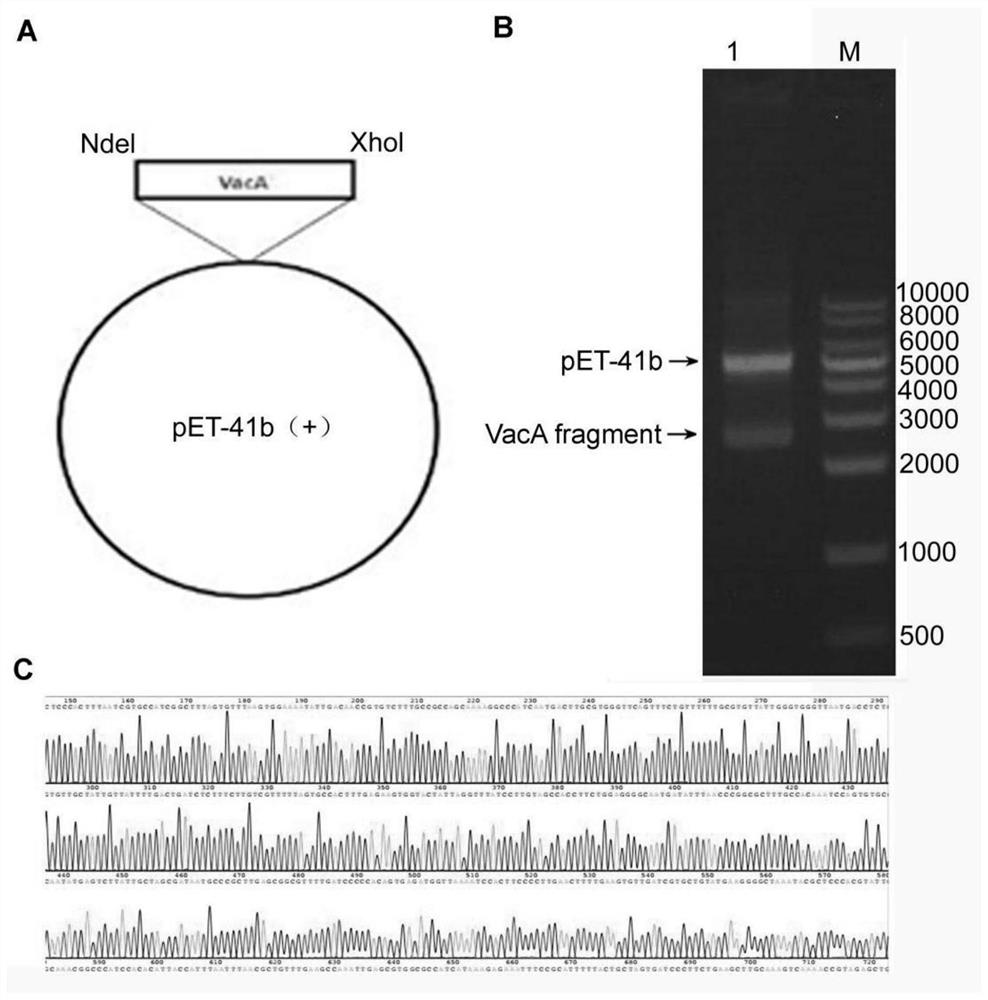
[0042] Construction of VacA recombinant expression vector
[0043]Using the vcaA gene sequence (VacA toxin mature fragment, the sequence of expressing the 34th-854th amino acid) of the H.pylori standard strain (strain J99 / ATCC 700824) as a template to synthesize mutation primers, primers at both ends of the gene, the primer sequence is as SEQ ID NO.1 and SEQ ID NO.2. The sequence SEQ ID NO.1 contains the recognition site of the restriction endonuclease Nde I, and the sequence SEQ ID NO.2 contains the recognition site of the restriction endonuclease Xho I. PCR amplification was carried out under the following conditions: 96°C for 5 min of pre-denaturation; 96°C for 30 seconds, 57°C for 30 seconds, 72°C for 1 minute and 20 seconds, and 72°C for 5 minutes (25 cycles).
[0044] After the PCR product was digested with restriction enzymes Nde I and Xho I, it was inserted into the expression vector plasmid pET41b (Novagen) containing a C-terminal histidine tag (8His tag); the PCR pr...
Embodiment 2
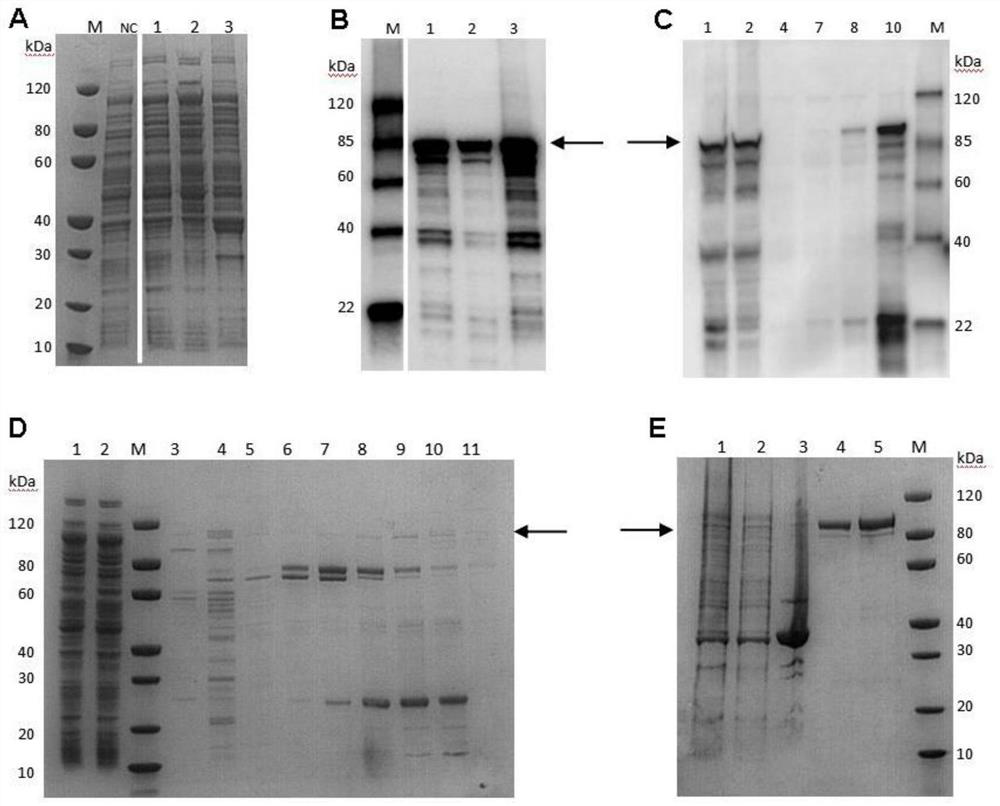
[0047] Expression and purification of VacA recombinant protein
[0048] The recombined plasmid was transformed into Escherichia coli BL21(DE3) and coated with K + Plates and incubated overnight at 37°C. A single colony was then inoculated into 50 ml of LB medium containing kanamycin (50 μg / ml) and the colonies were grown overnight at 37° C. in a shaker at 200 rpm. Take 100ul and transfer it to containing K + The cells were cultured in 5ml LB of 200 rpm and continued to be cultured at 37°C and 200rpm to observe the cell growth density. When the cell proliferation and growth curve reached OD=1.2 at 600nm, 0.5mM IPTG was added, and under different conditions (20°C for 20h, 37°C for induction 4h, 15°C induction for 16h) protein expression was induced at 200rpm. After induction, the bacteria were resuspended, and the expression was identified by SDS-PAGE and Western blot.
[0049] Collect the bacterial liquid obtained from the above culture, discard the supernatant after centri...
Embodiment 3
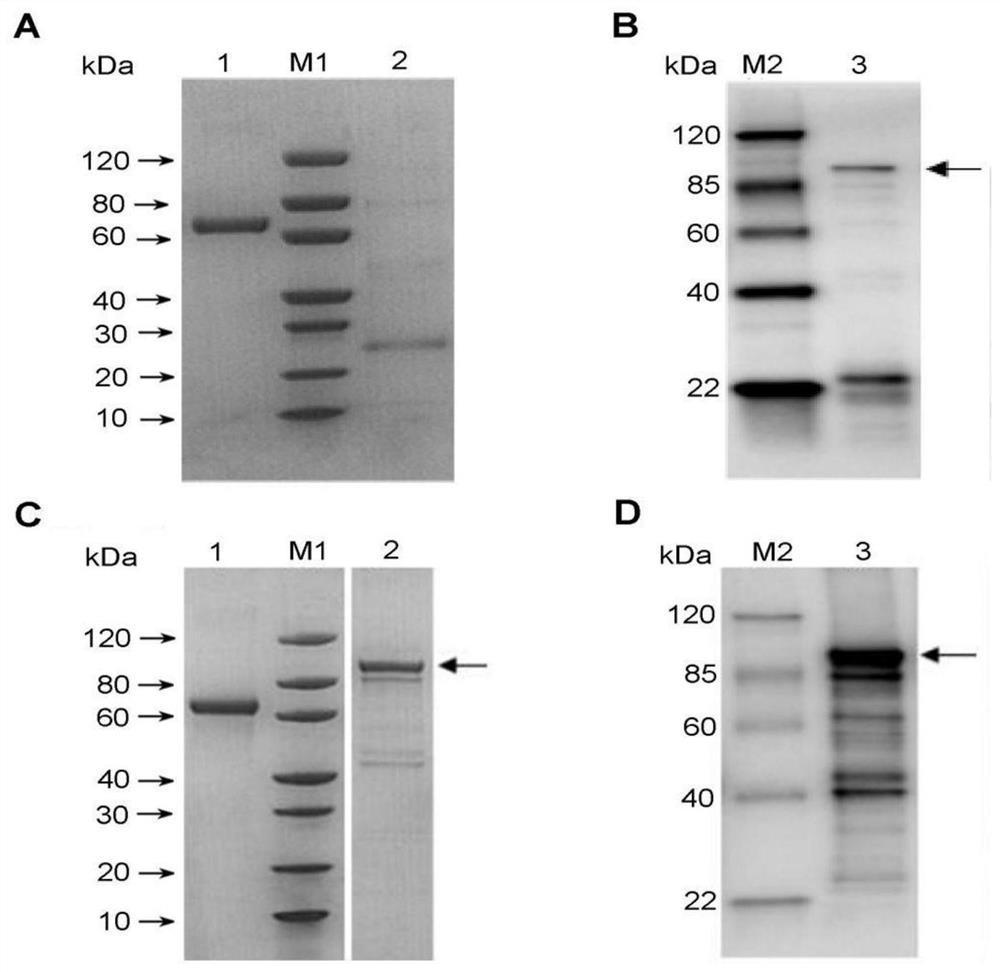
[0054] The VacA recombinant protein purified from inclusion bodies was renatured, and the buffer shown in Table 1 was selected. The recombinant protein was renatured in the buffer shown in Table 1, and the renaturation analysis of the protein was determined by the solubility of the protein (when the protein was dissolved in the buffer, if a precipitate appeared, it indicated that the renaturation failed, and if it was completely dissolved, it indicated that the renaturation failed. Sexual success), or according to the results of SDS-PAGE and Western-blot analysis (the lysate was collected, and the protein content was further analyzed by SDS-PAGE and Western-blot).
[0055] Table 1 Selection of renaturation buffer solution
[0056]
[0057]
[0058] The renatured VacA recombinant protein was collected, passed through a dialysis membrane with a molecular weight cut-off of 14 kDa for 4 hours, and then dialyzed with the same buffer mentioned above for 16 hours, and finally d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com