Method for analyzing impurities in mecobalamine sample
An analysis method, the technology of methylcobalamin, applied in the field of drug analysis, can solve the problems of inaccurate determination of the content of cyanocobalamin and hydroxocobalamin, unstable chemical properties, etc., and achieve the advantages of simple operation, good accuracy and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1 The recovery verification of the detection method provided by the application
[0090] Sample to be tested: Methylcobalamin raw material.
[0091] Chromatographic conditions: Octadecyl bonded silica gel is used as a filler, and the mobile phase is a mixed solution of 0.03mol / L potassium dihydrogen phosphate and methanol (that is, 0.03mol / L potassium dihydrogen phosphate is mobile phase A, methanol is mobile phase B), the column temperature is 40° C., the detection wavelength is 342 nm, and the solvent is 0.03 mol / L potassium dihydrogen phosphate solution. Gradient elution was carried out under the conditions shown in Table 1 below.
[0092] Table 1 Gradient elution program
[0093]
[0094]
[0095] Solution preparation:
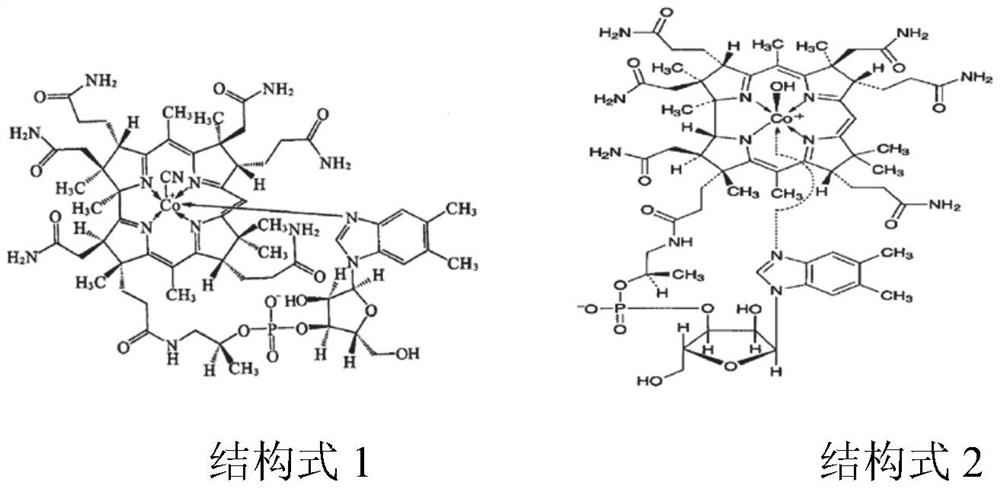
[0096] Impurity stock solution: take hydroxocobalamin acetate and cyanocobalamin, accurately weigh, add solvent (0.03mol / L potassium dihydrogen phosphate solution) to dissolve and quantitatively dilute to make hydroxocobalamin, cyan...
Embodiment 3
[0108] Cyanocobalamin, hydroxocobalamin impurity detection result in the methylcobalamin raw material of embodiment 3
[0109] Chromatographic conditions: Octadecyl bonded silica gel is used as a filler, and the mobile phase is a mixed solution of 0.03mol / L potassium dihydrogen phosphate and methanol (that is, 0.03mol / L potassium dihydrogen phosphate is mobile phase A, methanol is mobile phase B), the column temperature is 40° C., the detection wavelength is 342 nm, and gradient elution is performed under the conditions shown in Table 4 below.
[0110] Table 4 Gradient elution program
[0111]
[0112]
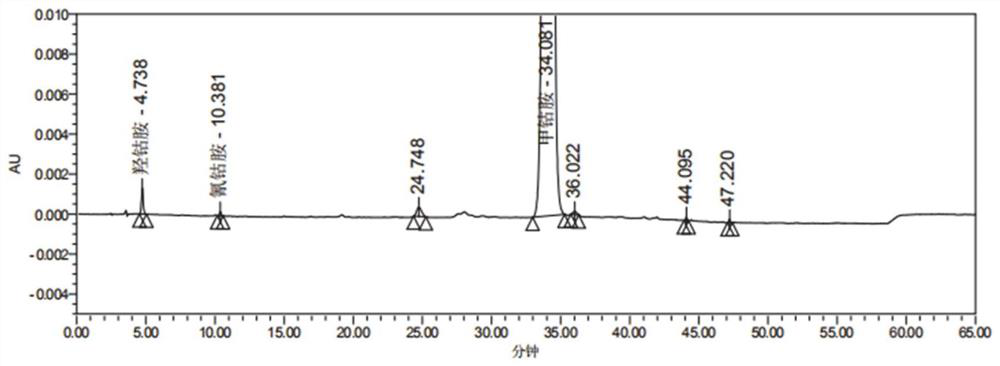
[0113] Preparation of solution: take the methylcobalamin raw material and prepare a test sample with a concentration of 0.5 mg / ml with 0.03 mol / L potassium dihydrogen phosphate solution. Take 20ul and inject into the liquid chromatograph, record the chromatogram as figure 1 Shown, retention time 4.783min is hydroxocobalamin absorption peak, retention time 10.381min is ...
Embodiment 4
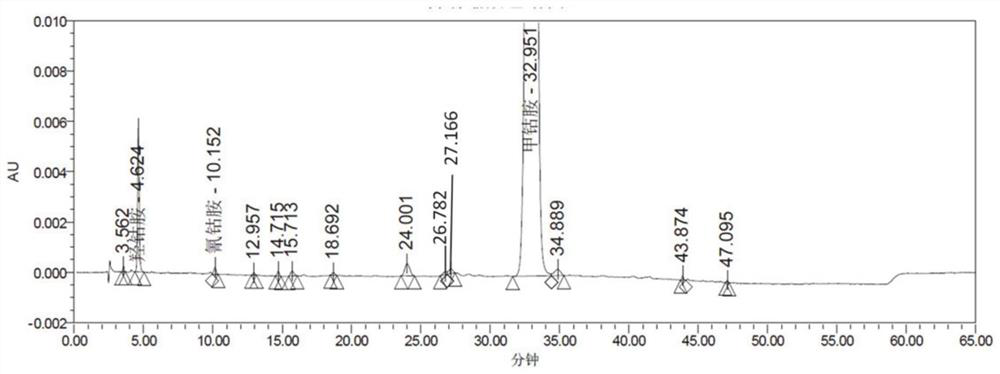
[0114] Cyanocobalamin, hydroxocobalamin impurity detection result in embodiment 4 methylcobalamin tablets
[0115] Chromatographic conditions: Octadecyl bonded silica gel is used as a filler, and the mobile phase is a mixed solution of 0.03mol / L potassium dihydrogen phosphate and methanol (that is, 0.03mol / L potassium dihydrogen phosphate is mobile phase A, methanol is mobile phase B), the column temperature is 40° C., the detection wavelength is 342 nm, and gradient elution is performed under the conditions shown in Table 5 below.
[0116] Table 5 Gradient elution program
[0117] time (minutes) Mobile phase A(%) Mobile phase B(%) 0 90 10 10 86 14 35 85 15 45 75 25 55 75 25 55.01 90 10 65 90 10
[0118] Solution preparation: Take methylcobalamin tablets, grind into fine powder, and prepare a sample with a concentration of 0.5 mg / ml (calculated as methylcobalamin) with 0.03mol / L potassium dihydrogen phosphate solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com