Early screening method and kit for non-alcoholic fatty liver disease susceptibility genes
A fatty liver disease and susceptibility gene technology, applied in the field of early screening methods and kits for non-alcoholic fatty liver disease susceptibility genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
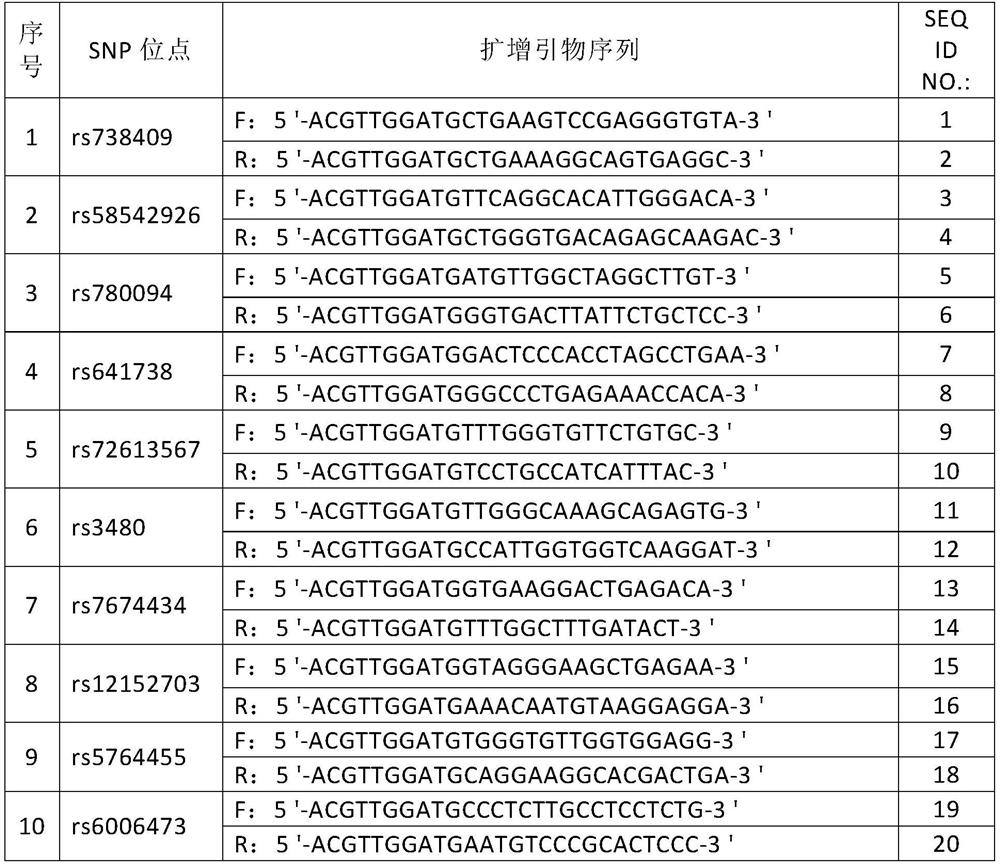
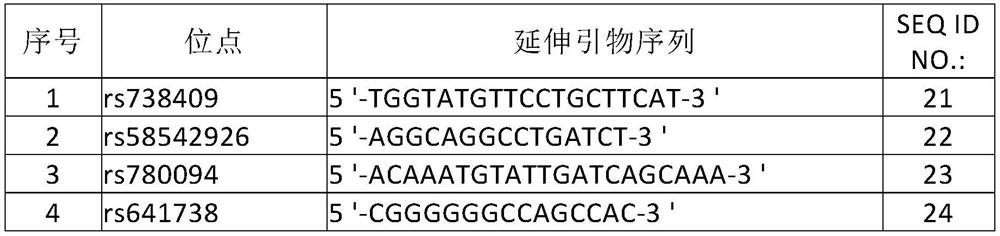
[0093] Feasibility analysis of SNP site screening of human non-alcoholic fatty liver disease susceptibility-related genes
[0094] By searching NCBI home and abroad genome-wide association study (genome-wide association study, GWAS) in large-scale pathological control group clinical research of the relevant sites of non-alcoholic fatty liver disease, the inventors screened and Evaluation, selected 10 single nucleotide polymorphism sites that are significantly associated with the risk of non-alcoholic fatty liver disease in the Chinese population, and are independent of each other, there is no linkage disequilibrium, so the site selection of the present invention is representative , independence and cumulative risk value, which can be used to assess the risk of individuals suffering from non-alcoholic fatty liver disease.
[0095] The screened SNP sites are as follows:
[0096]rs738409, rs58542926, rs780094, rs641738, rs72613567, rs3480, rs7674434, rs12152703, rs5764455, rs600...
Embodiment 2
[0097] Embodiment 2 system verification
[0098] System validation includes accuracy, specificity, sensitivity, precision, and comparison among personnel.
[0099] Accuracy verification scheme: 20 cases were detected at each site, compared with Sanger sequencing, the expected target was 95%.
[0100] Specificity Validation Protocol: Included in Accuracy, expected target 95%.
[0101] Sensitive verification scheme: using human genomic DNA positive samples as templates, the DNA contents of calibration samples were 1ng / μL, 5ng / μL, 10ng / μL, 50ng / μL, and 100ng / μL for sensitivity inspection.
[0102] The precision verification plan (including intra-batch, inter-batch, and personnel comparisons, not involving inter-instrument comparisons) has an expected target of 95%.
[0103] Intra-assay precision: The same batch of each sample was repeated 3 times to compare the intra-assay precision.
[0104] Inter-batch precision: The same operator tests the same sample in multiple batches to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com