Patents
Literature
45results about How to "Good technical reproducibility" patented technology
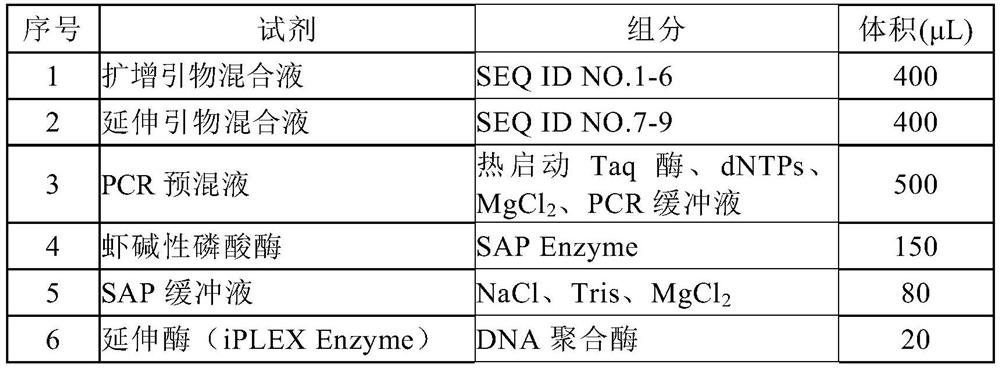
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
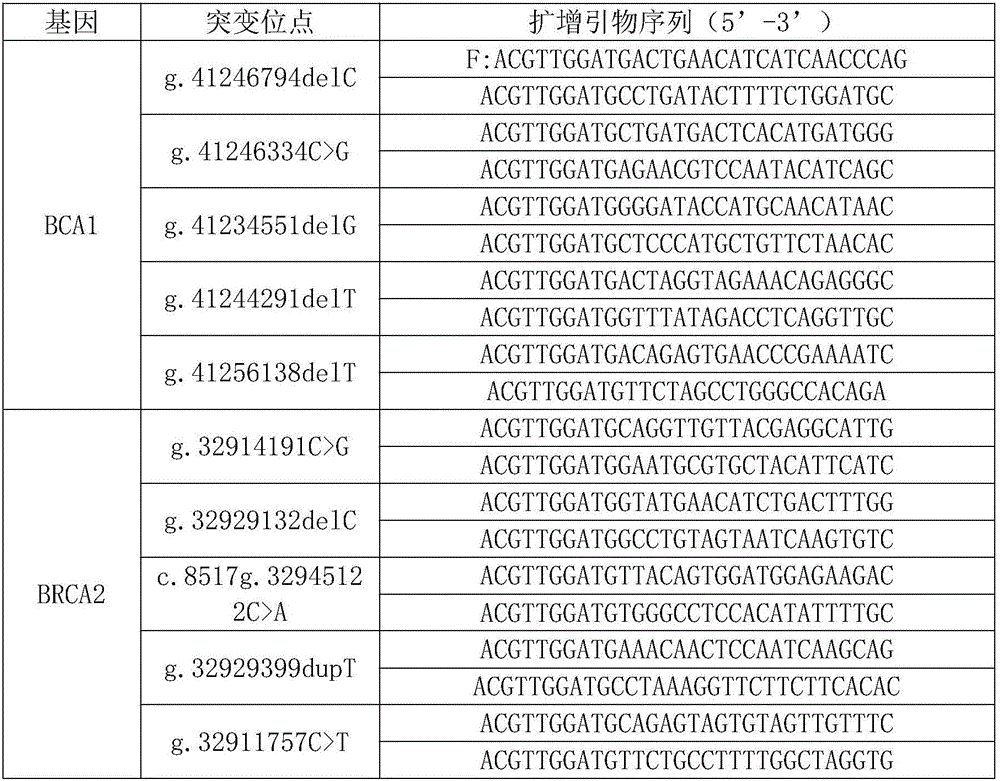
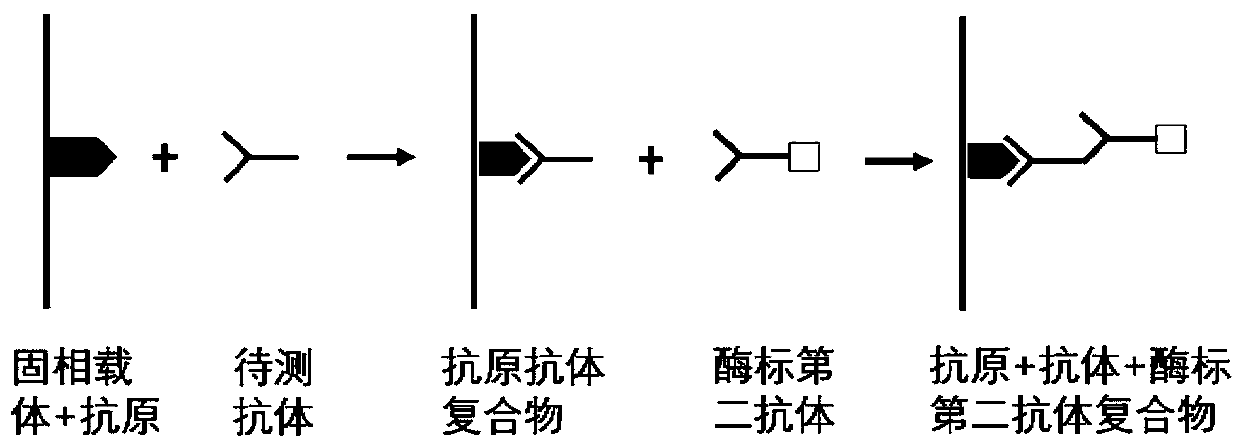
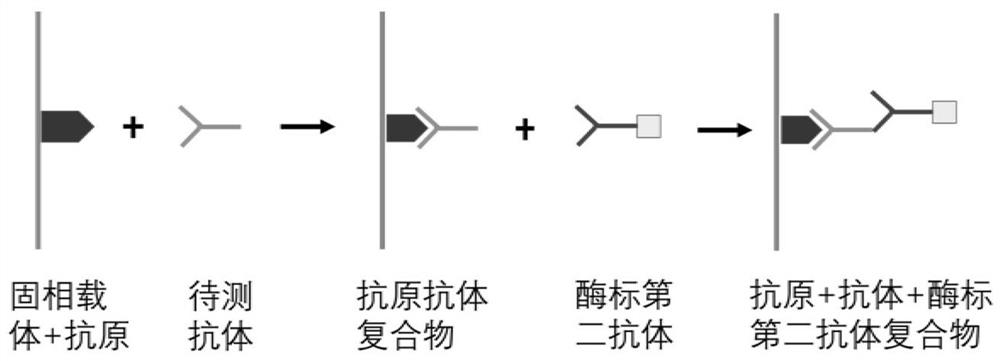
Autoantibody joint detection ELISA kit for screening early esophageal cancer
ActiveCN110187108AEfficient detectionHigh detection sensitivityMaterial analysisHigh risk populationsElisa kit
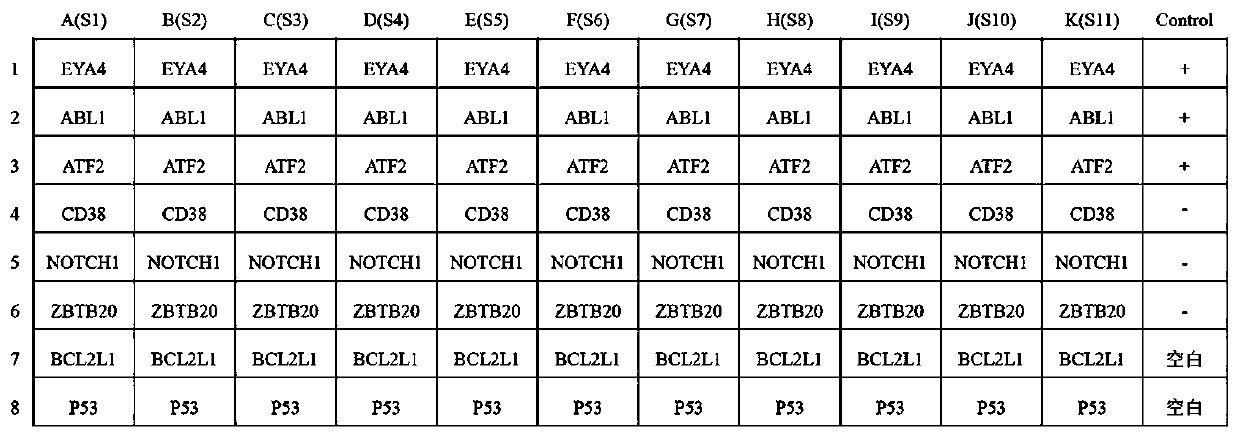
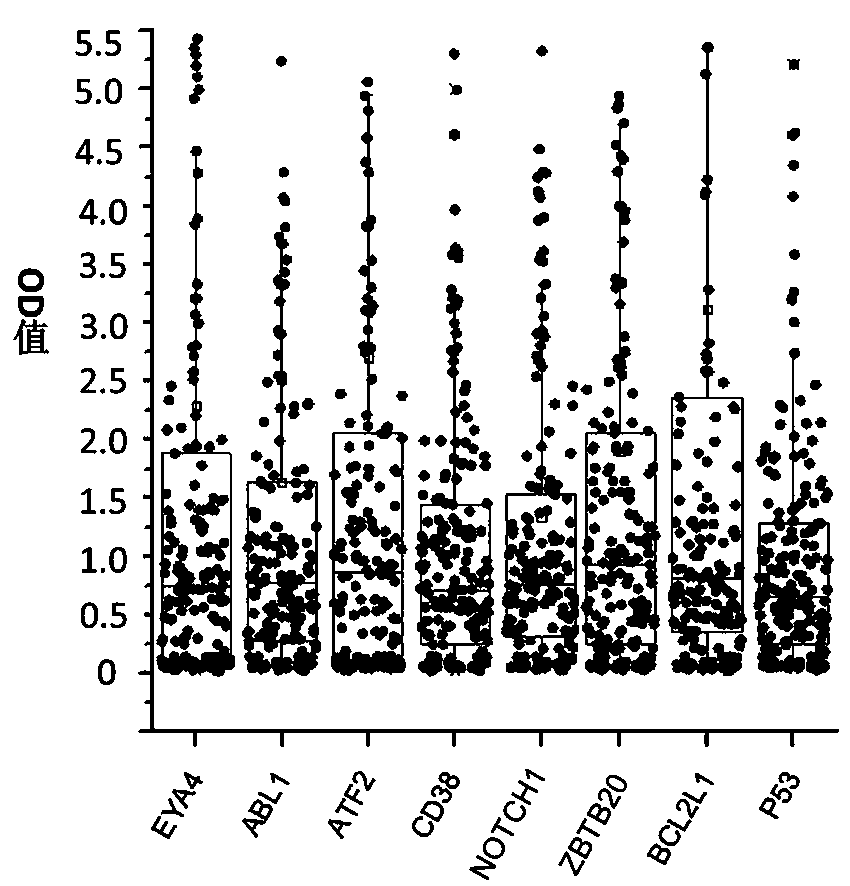
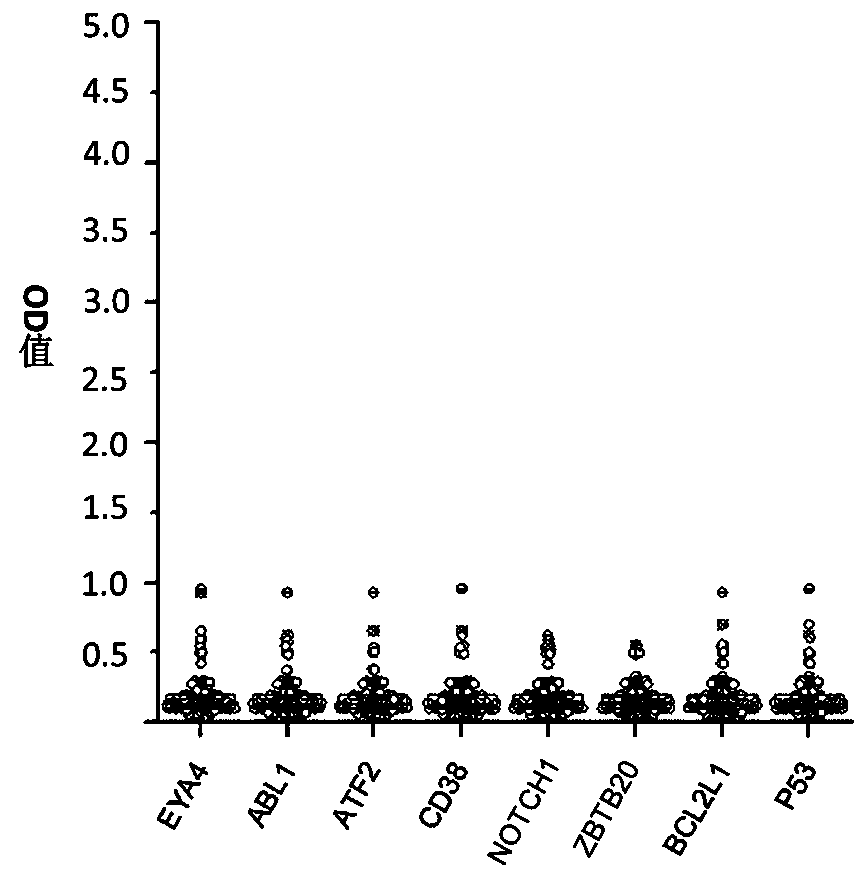
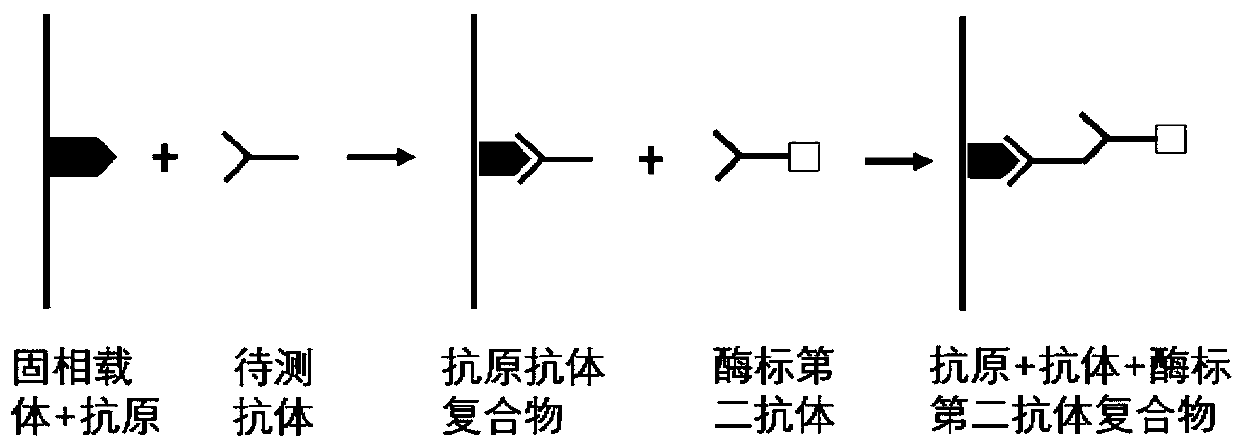
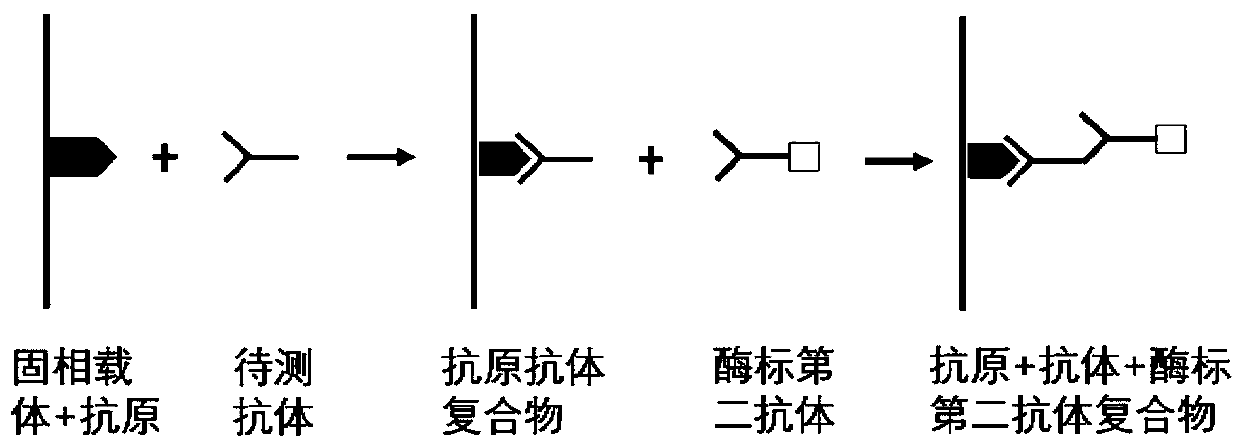
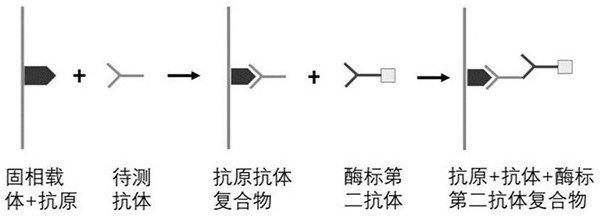
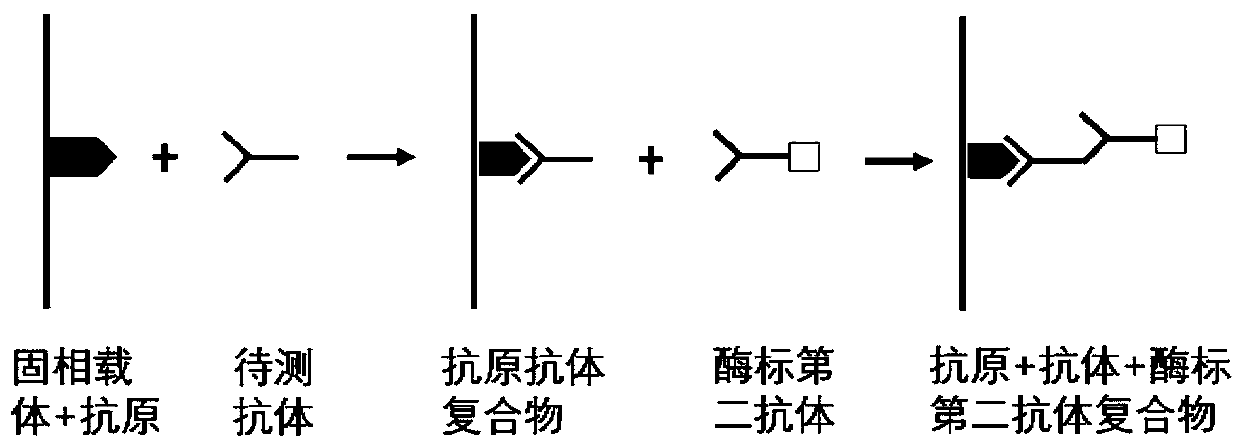
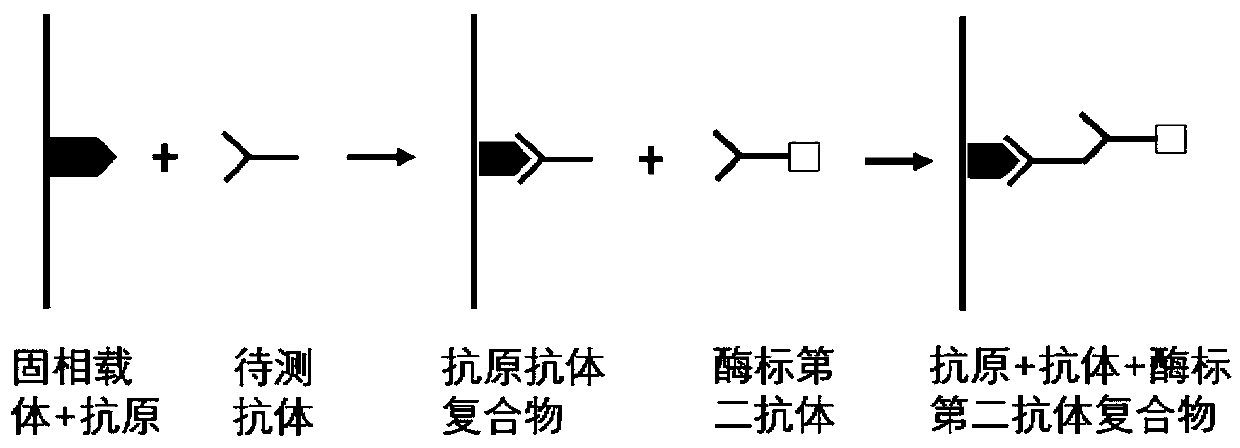
The invention belongs to the technical field of tumor medicine, and particularly discloses an autoantibody joint detection ELISA kit for screening early esophageal cancer. The kit comprises a solid-phase carrier and tumor-associated antigens coated on the solid-phase carrier, wherein the tumor-associated antigens are EYA4, ABL1, ATF2, CD38, NOTCH1, ZBTB20, BCL2L1 and P53. Furthermore, the kit alsocomprises a sample diluent, a second antibody, a second antibody diluent, positive control serum, negative control serum, a color developing solution, a stop solution and a washing solution. The ELISA kit provided by the invention can effectively detect esophageal cancer, especially early esophageal cancer, has the detection sensitivity of 94% and the specificity of 79%, can be used for large-scale screening of asymptomatic people in high-incidence areas of esophageal cancer, and is beneficial to screening and early discovery of asymptomatic high-risk population.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV +1
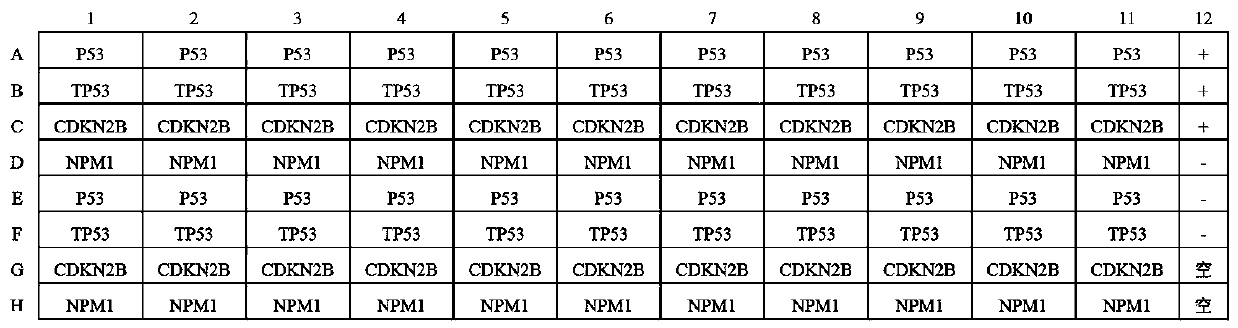
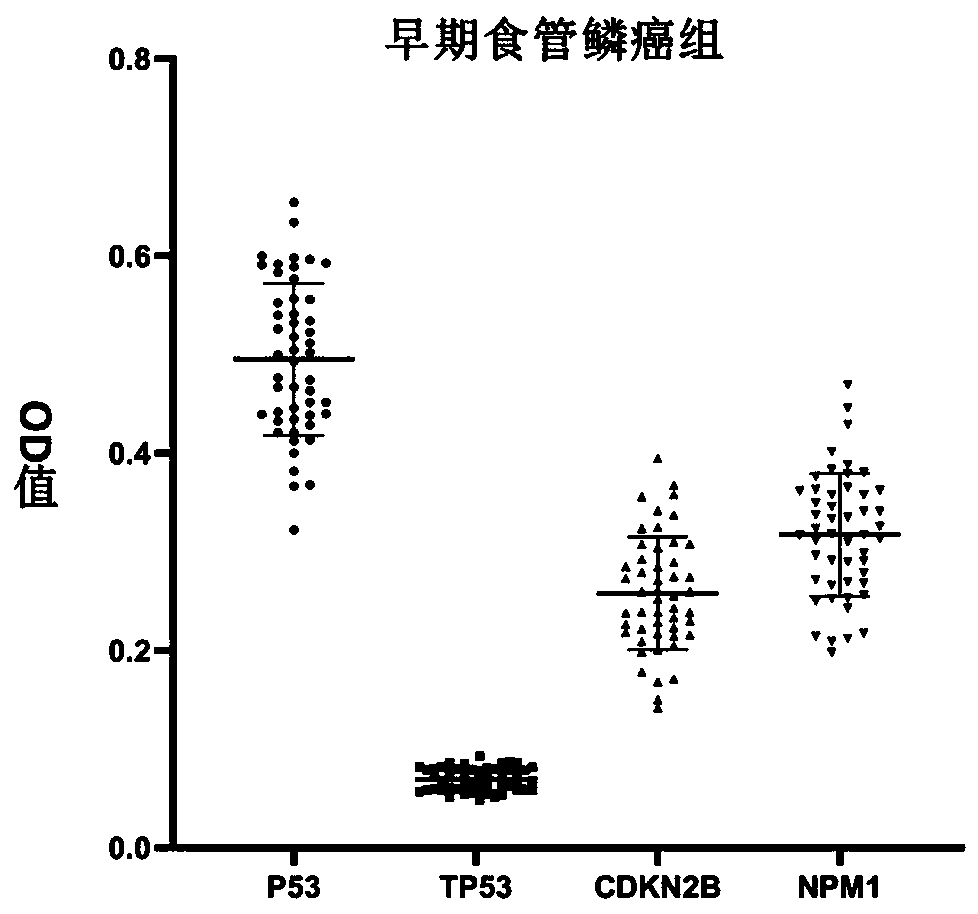
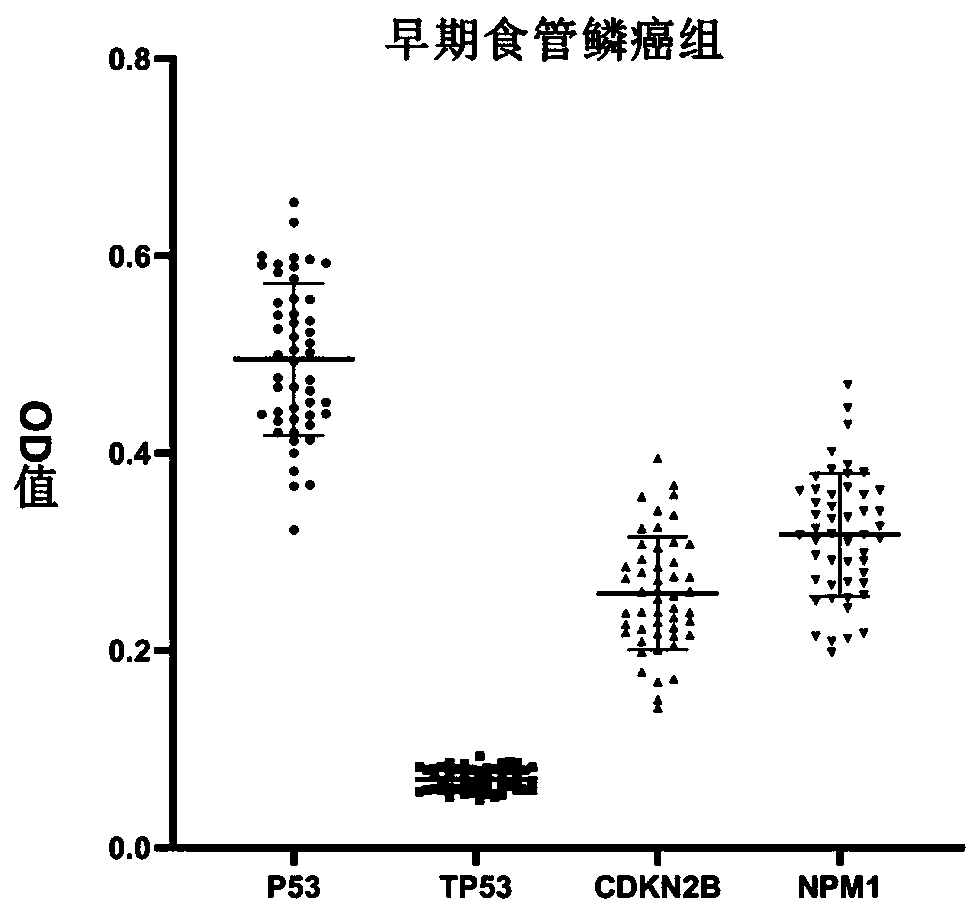
Autoantibody joint detection ELISA kit for early screening of esophageal squamous carcinoma
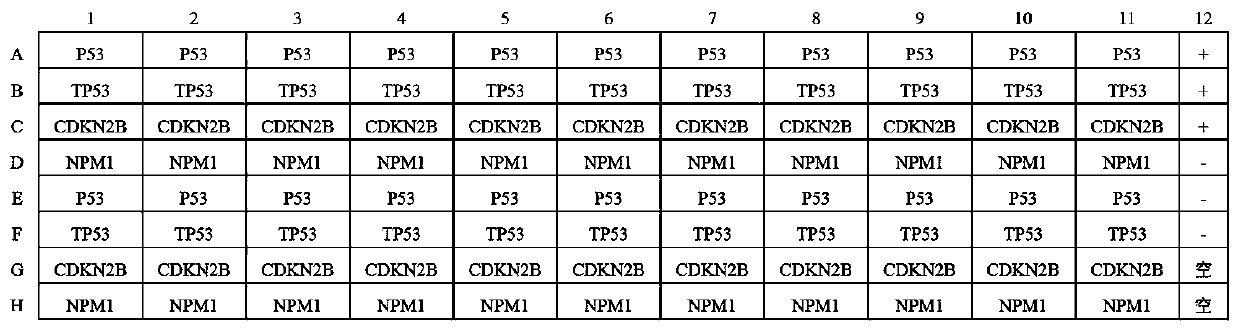
The invention belongs to the technical field of medical oncology, and particularly discloses an autoantibody joint detection ELISA kit for early screening of esophageal squamous carcinoma. The kit comprises a solid-phase carrier and a tumor-associated antigen coated on the solid-phase carrier, wherein the tumor-associated antigen consists of P53, TP53, CDKN2B and NPM1. Furthermore, the kit comprises sample diluent, a second antibody, second antibody diluent, positive control serum, negative control serum, developing solution, stop solution and washing solution. The ELISA kit is capable of effectively detecting the esophageal squamous carcinoma, especially the early esophageal squamous carcinoma, has detection sensitivity up to 88.8% and specificity up to 86.3%, can be used for the large-scale screening of asymptomatic groups in high-risk areas of the esophageal squamous carcinoma, and is beneficial for the esophageal squamous carcinoma screening and early discovery of the asymptomatichigh-risk groups.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
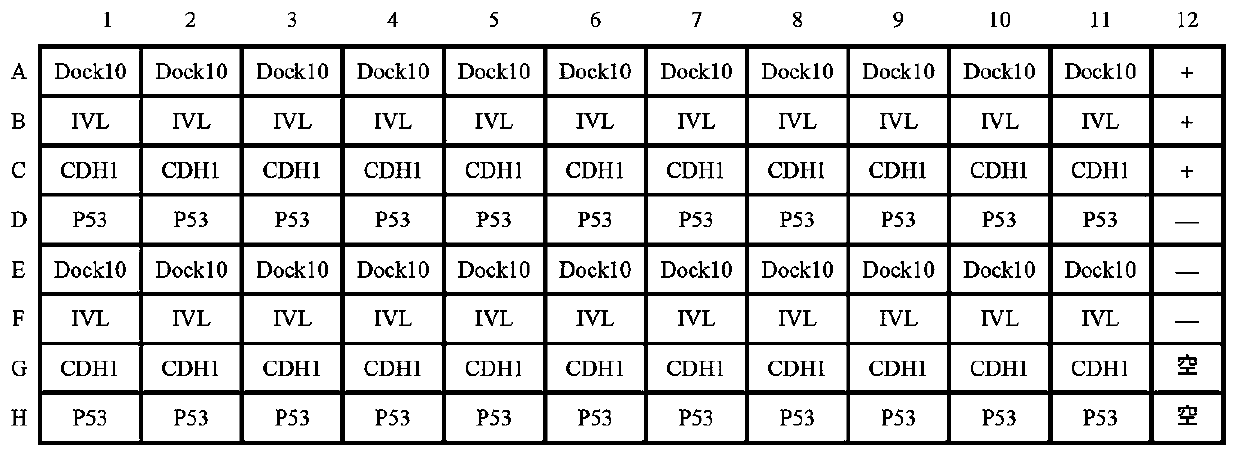
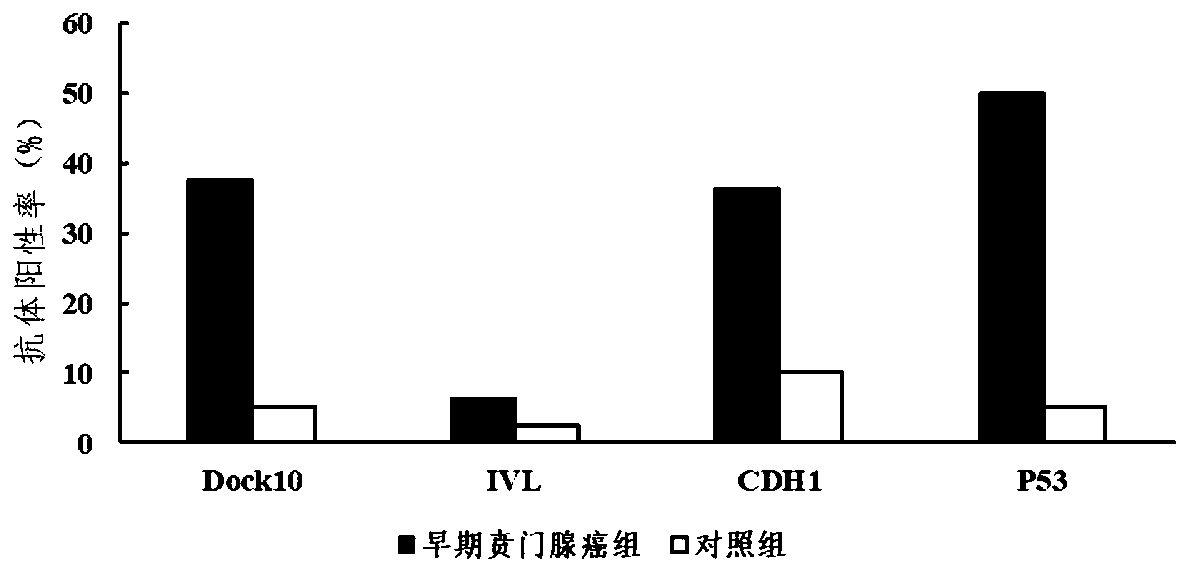
Autoantibody joint detection ELISA kit for gastric cardiac adenocarcinoma early screening
The invention belongs to the technical field of the tumor medicine, and specifically discloses an autoantibody joint detection ELISA kit for gastric cardiac adenocarcinoma early screening. The kit comprises a solid-phase carrier and tumor-associated antigen coated on the solid-phase carrier; the tumor-associated antigen is composed of Dock10, IVL, CDH1 and P53; furthermore, the kit further comprises sample diluent, a second antibody, second antibody diluent, positive control serum, negative control serum, a developing solution, a stop solution and a washing solution. The ELISA kit disclosed bythe invention can effectively detect the gastric cardiac adenocarcinoma, especially the early gastric cardiac adenocarcinoma; the detection sensitivity is up to 87.5%, and the specificity reaches 75%; and the kit can be used for the large-scale screening of the asymptomatic population at the high incidence area of the gastric cardiac adenocarcinoma, thereby facilitating the gastric cardiac adenocarcinoma screening and early discovery of the asymptomatic high-risk group.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
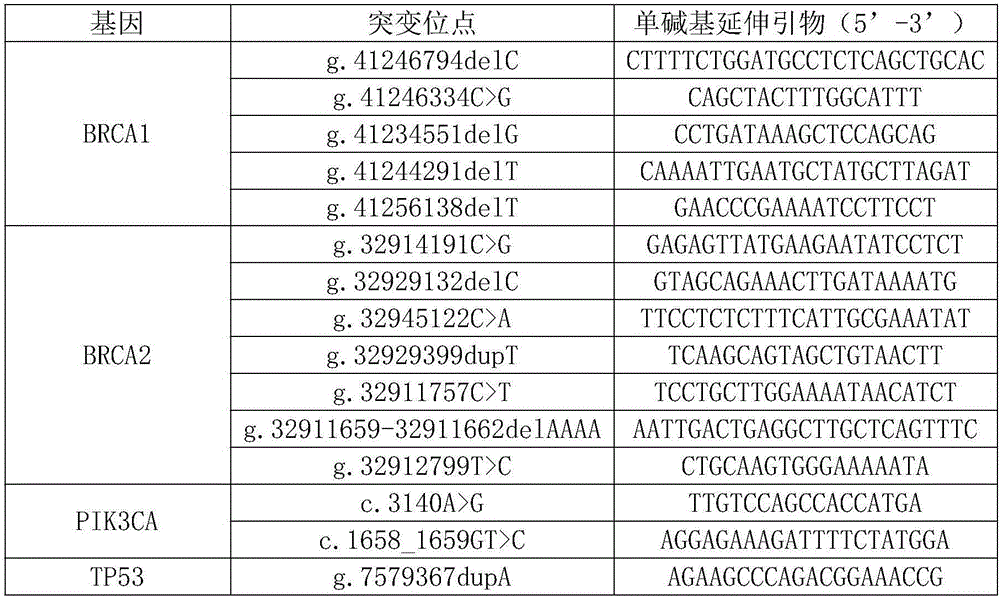
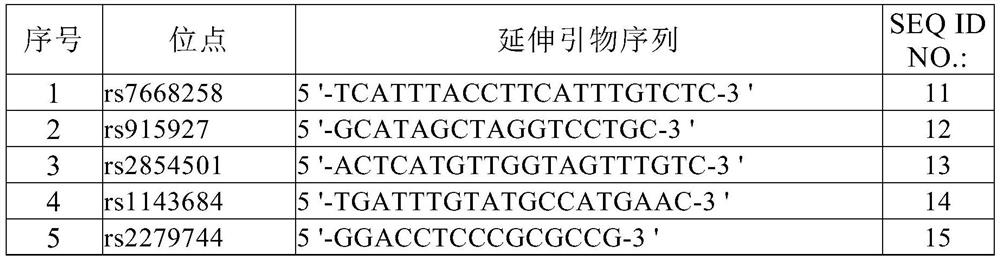
Kit for detecting gastric cancer susceptibility and SNP marker thereof
InactiveCN106434979AImprove detection success rateEarly Assessment ImplementationMicrobiological testing/measurementDNA/RNA fragmentationGastric carcinomaObserved Survival
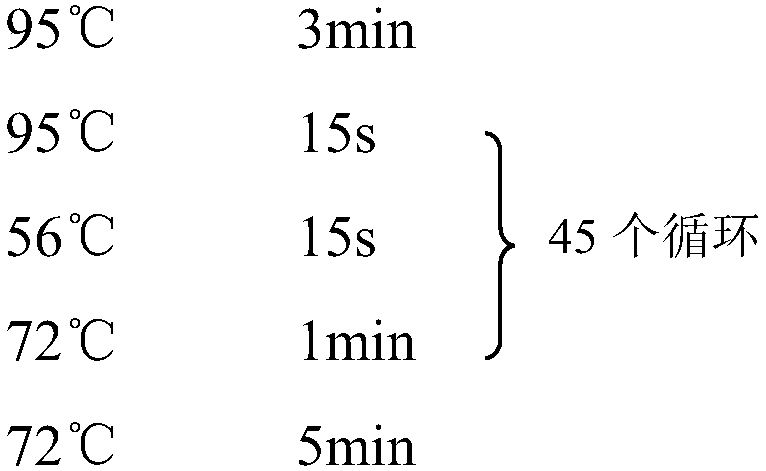
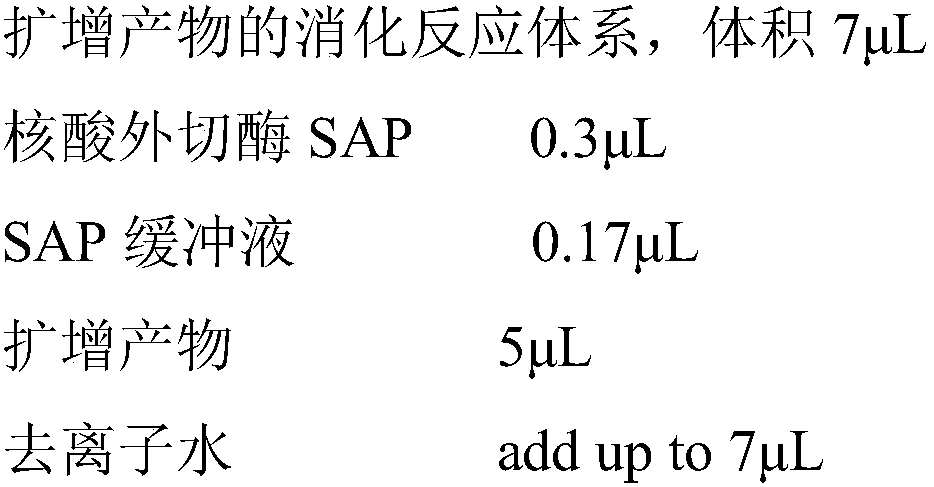
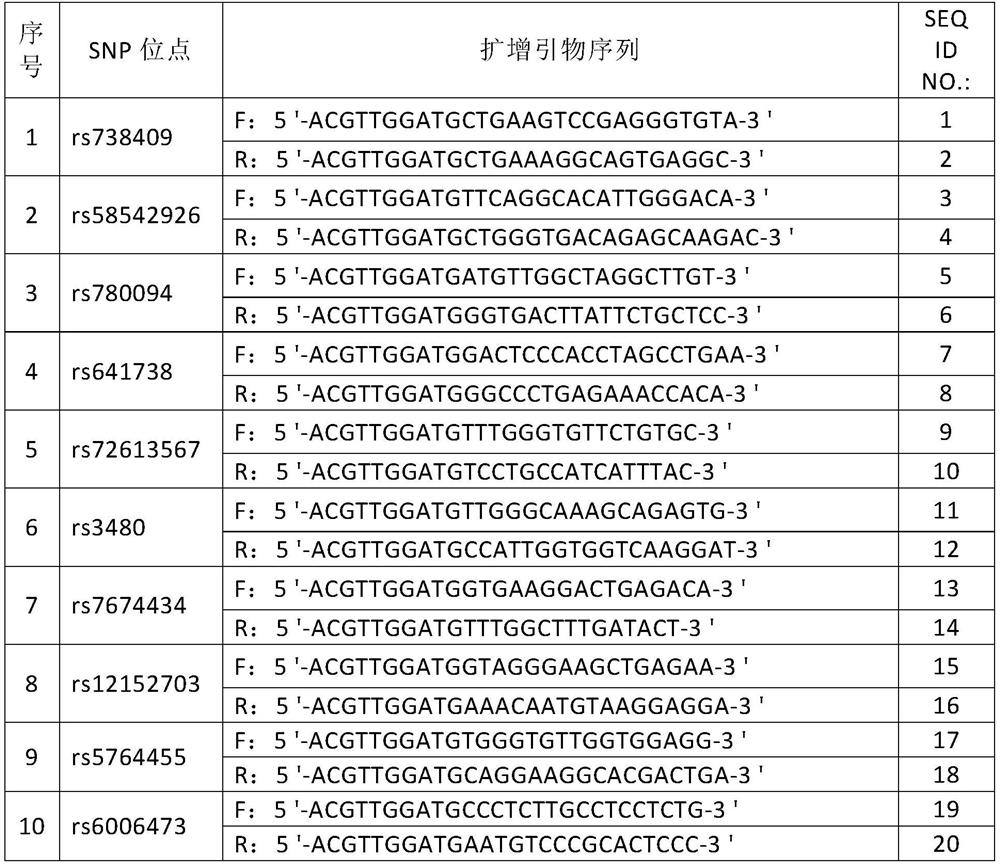
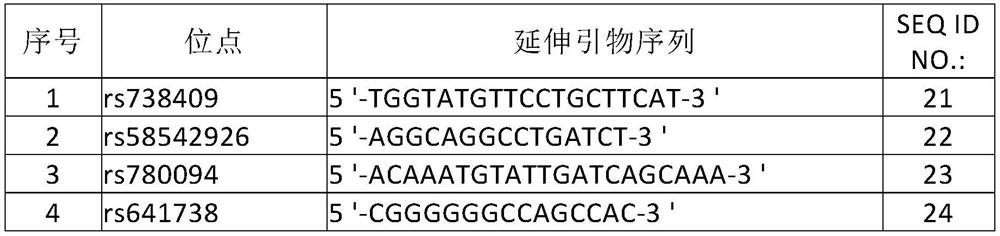
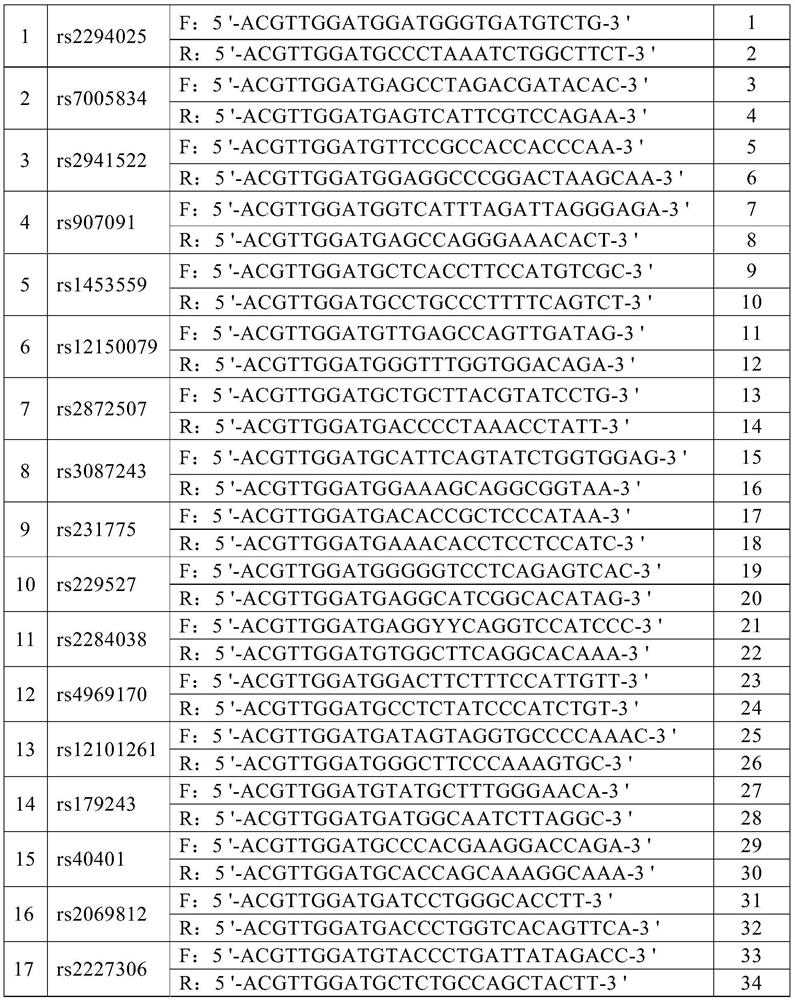
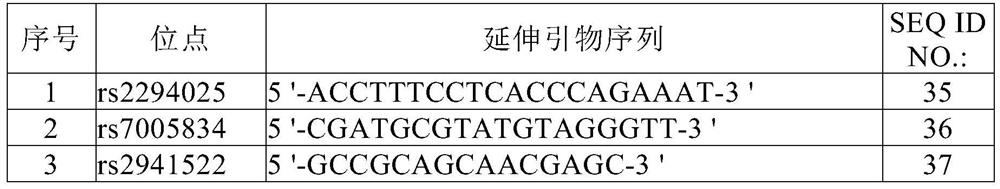
The invention discloses an SNP marker for detecting gastric cancer susceptibility. The SNP marker comprises 28 SNP loci. The invention further discloses a PCR (polymerase chain reaction) amplification primer, a single base extension primer and a kit of the SNP marker. Literature demonstrates that the 28 SNP loci have high practicability, can be used for early evaluation and large-scale screening of gastric cancer and are high in detection survival rate, technological repeatability and performance-cost ratio, and an important basis is provided for disease risk evaluation and diagnosis reference of the gastric cancer.
Owner:深圳市核子基因科技有限公司
Detecting method of nucleic acid mass spectrum for liver cancer susceptibility gene early screening
ActiveCN107557461AImprove detection success rateGood value for moneyMicrobiological testing/measurementSmall sampleNucleotide
The invention relates to the field of gene detection, in particular to a detecting method of a nucleic acid mass spectrum for liver cancer susceptibility gene early screening. The detecting method ischaracterized by screening out a combination of SNPs (Single Nucleotide Polymorphisms) of susceptibility gene related to liver cancer of Chinese people and gene mutation sites related to the liver cancer by considering the difference between liver cancer gene spectra of Chinese population and European and American populations, and widely (high-throughput detecting sites and high-throughput detecting samples) screening and checking genetic markers related to the liver cancer by utilizing a nucleic acid mass spectrometer. According to the detecting method disclosed by the invention, the detection success rate is high, the technology reproducibility is good, the cost performance ratio is high, polygenic detection of a single small sample can be realized, and maximum use of the small sample can be met. The detecting method is based on MassARRAY nucleic acid spectrometry; compared with Sanger sequencing, the detecting method has the technical advantages of high accuracy and high sensitivity, the detecting result is stable, and the detection positive rate is increased.
Owner:武汉赛云博生物科技有限公司
Early screening method and kit for non-alcoholic fatty liver disease susceptibility genes
ActiveCN113025701AImprove detection success rateGood technical reproducibilityMicrobiological testing/measurementDNA/RNA fragmentationMass analyzerRelated gene
The invention provides an early screening method and a kit for non-alcoholic fatty liver disease susceptibility genes. In particular, the difference of non-alcoholic fatty liver disease gene spectrums of Chinese people and European and American people is considered, and a combination of SNP loci of genes related to non-alcoholic fatty liver disease susceptibility of Chinese people is screened out; wide (high-throughput detection sites and high-throughput detection samples) screening and inspection are carried out on genetic markers related to the non-alcoholic fatty liver disease by using a nucleic acid mass spectrometer. The method disclosed by the invention is high in detection success rate, good in technical reproducibility and high in cost performance, can realize multi-gene detection of a single small sample, and meets maximum use of the small sample; The method has the technical advantages of high accuracy and high sensitivity, the detection result is stable, and the detection positive rate is increased.
Owner:北京科力丹迪生物医疗科技有限公司
Method of detecting breast cancer early diagnosis related genes based on nucleic mass spectrometry
InactiveCN107523646AEarly screening is accurateGood technical reproducibilityMicrobiological testing/measurementDNA/RNA fragmentationBreast cancer susceptibility genesProtein mass spectrometry
The invention belongs to the field of gene detection, and in particular relates to a method for detecting genes related to early diagnosis of breast cancer based on nucleic acid mass spectrometry technology. The present invention provides a combined detection method for 15 breast cancer-related mutation sites of 4 genes and 18 breast cancer susceptibility SNP sites of 10 genes, and uses SequenomMassARRAYSNP genotype analysis technology to detect SNPs of breast cancer susceptibility genes Genotyping detection of loci and gene mutation loci associated with breast cancer. This method is more accurate for early screening of breast cancer, has good technical reproducibility, and is cost-effective; compared with TaqMan-PCR and SNP detection of second-generation platform sequencing, it has flexible design, high accuracy, and large sample size of detection sites , low cost and other advantages, hundreds to thousands of samples can be tested on dozens of SNP sites at the same time.
Owner:武汉赛云博生物科技有限公司
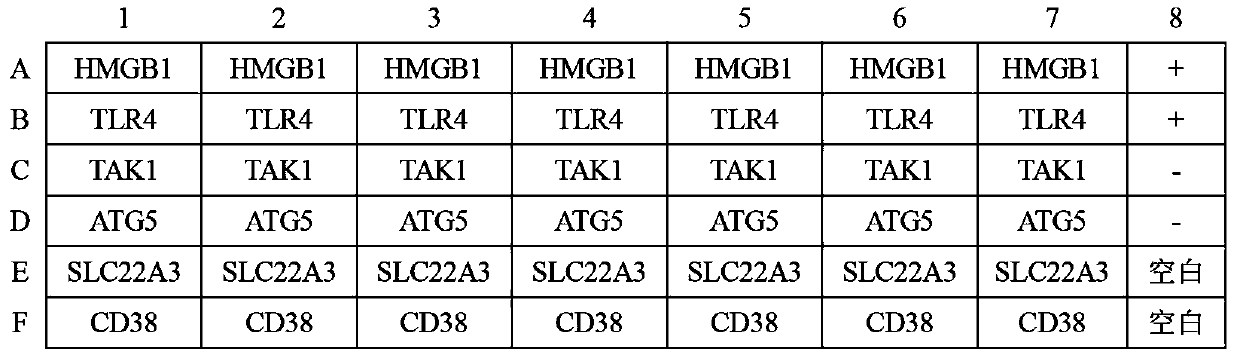
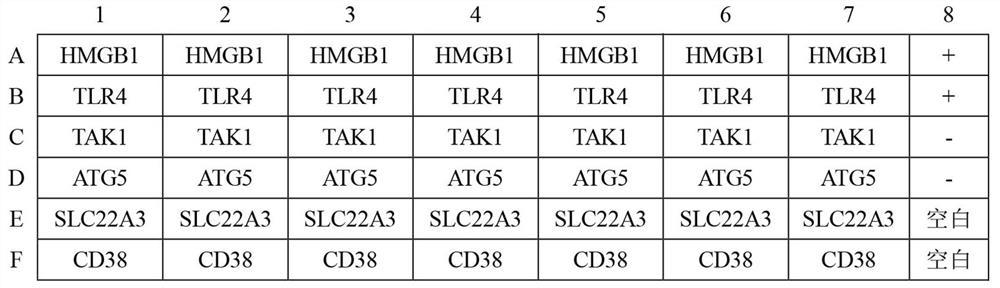
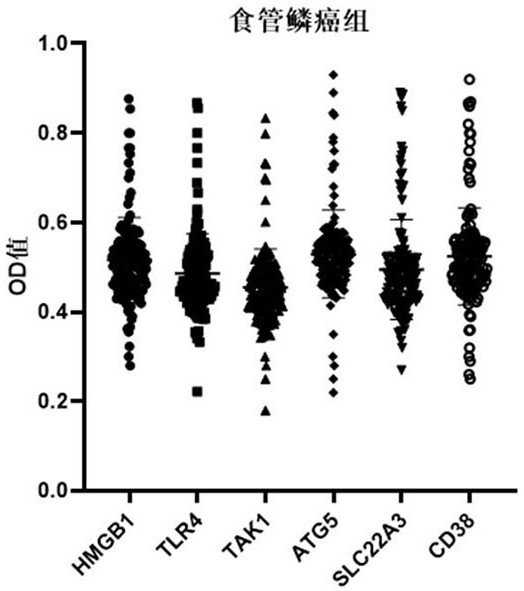
ELISA kit for early diagnosis of esophageal squamous cell carcinoma
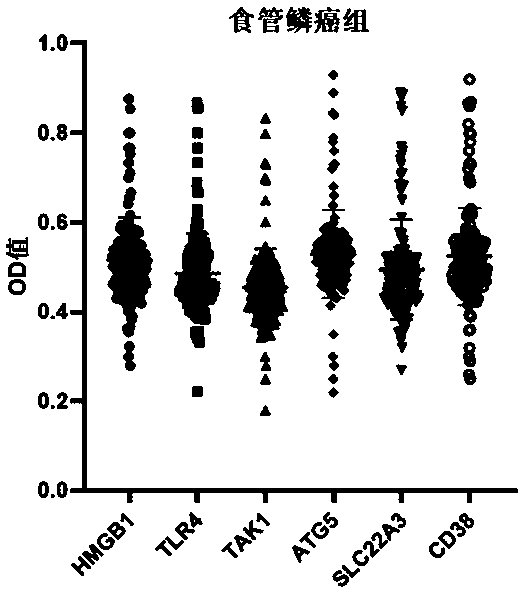
The invention belongs to the technical field of medical biology, and particularly discloses an ELISA kit for early diagnosis of esophageal squamous cell carcinoma. The kit comprises a solid phase carrier and a tumor-associated antigen coated on the solid phase carrier, and the tumor-associated antigen is composed of HMGB1, TLR4, TAK1, ATG5, SLC22A3 and CD38. Furthermore, the kit also comprises a sample diluent, a second antibody, a second antibody diluent, positive control serum, negative control serum, a developing solution, a stop solution and a washing solution. The ELISA kit provided by the invention can effectively detect esophageal squamous cell carcinoma, especially early esophageal squamous cell carcinoma, has detection sensitivity as high as 92.7% and specificity as high as 84.0%,can be used for large-scale screening and diagnosis of asymptomatic people in esophageal squamous cell carcinoma high-incidence areas, and is beneficial to screening and early discovery of asymptomatic high-risk people.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Kit for detecting susceptibility to lung cancer and SNP (Single Nucleotide Polymorphism) marker thereof
InactiveCN106434978AImprove detection success rateEarly Assessment ImplementationMicrobiological testing/measurementDNA/RNA fragmentationGeneticsLung cancer
The invention discloses an SNP (Single Nucleotide Polymorphism) marker for detecting the susceptibility to lung cancer. The SNP marker comprises 36 SNP loci. The invention further discloses a PCR (Polymerase Chain Reaction) amplification primer and a single-base extension primer of the SNP marker as well as a kit of the SNP marker. An important basis is provided for prevalence risk assessment and diagnostic reference of the lung cancer, so that early diagnosis of the lung cancer is realized.
Owner:深圳市核子基因科技有限公司
Kit for detecting nasopharyngeal cancer susceptibility and SNP marker of kit
InactiveCN106755318AImprove detection success rateEarly Assessment ImplementationMicrobiological testing/measurementDNA/RNA fragmentationNasopharyngeal cancerAssessment diagnosis
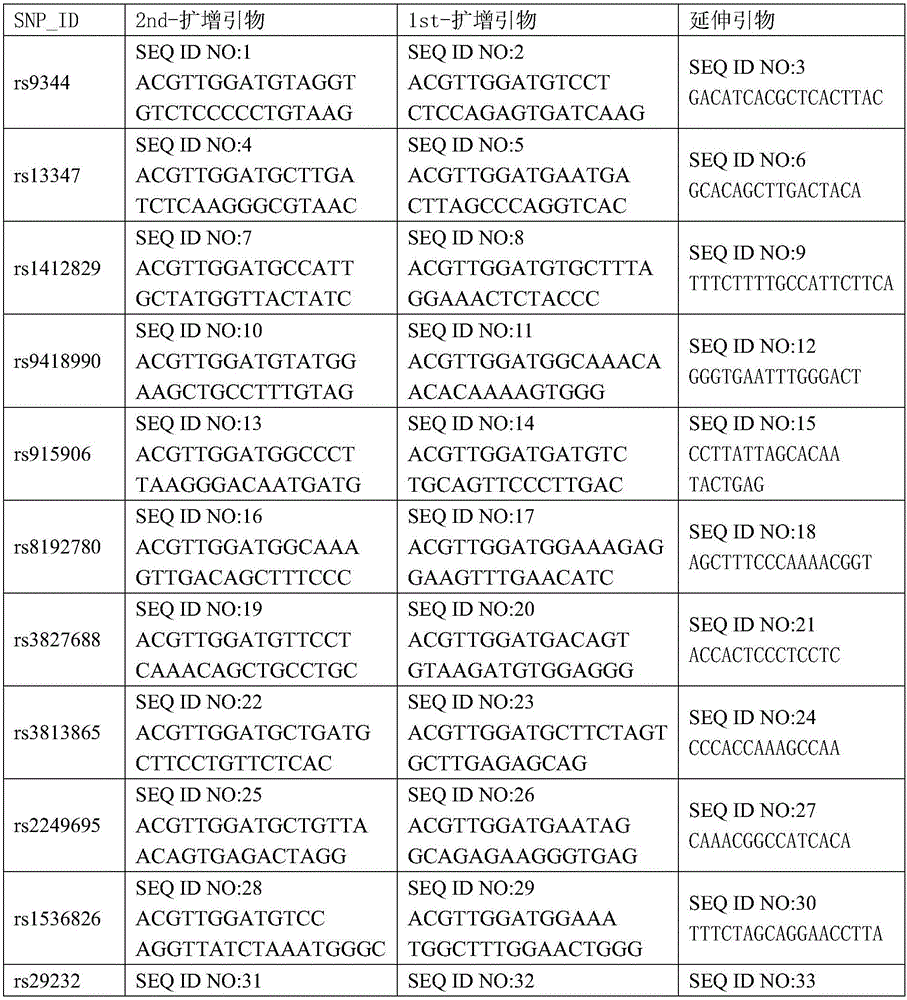
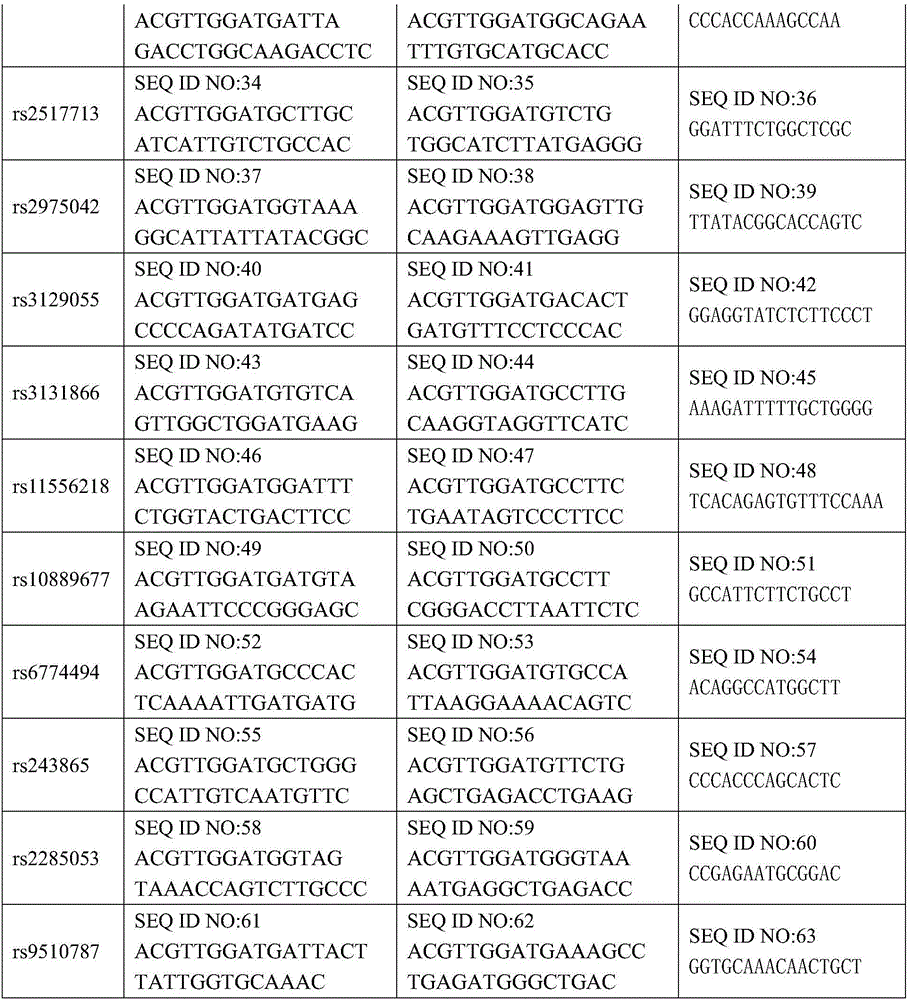
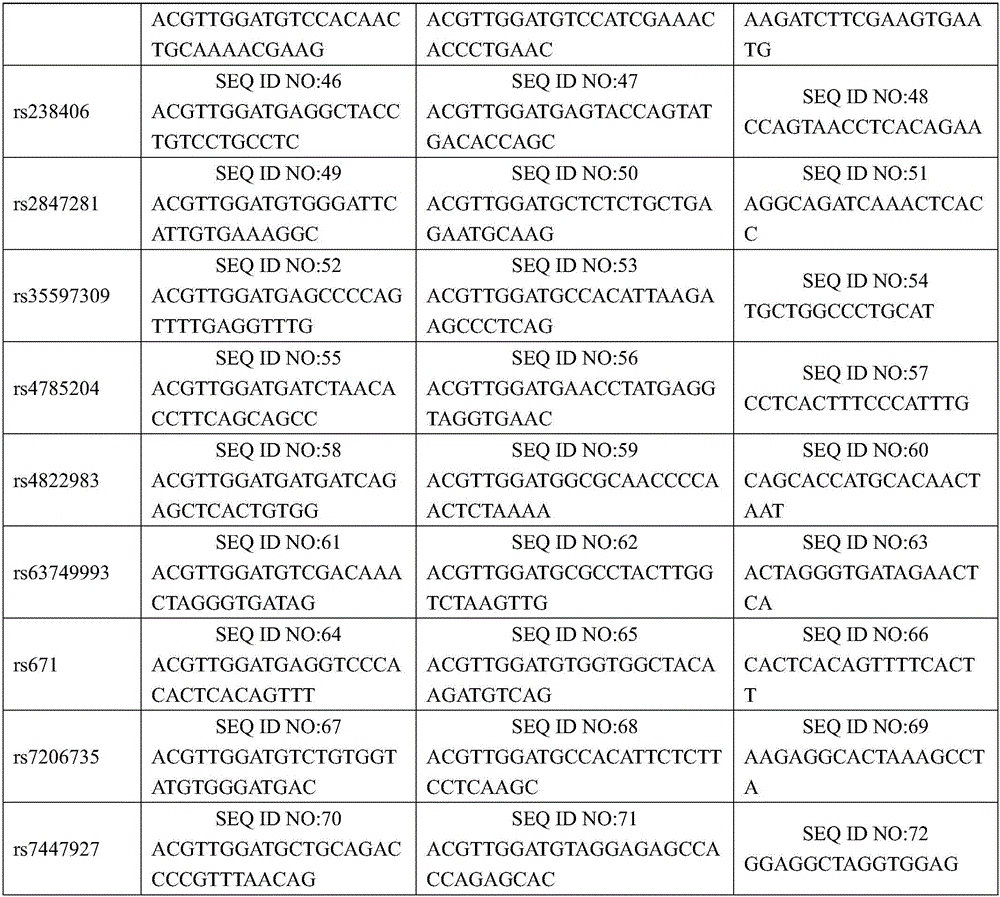
The invention discloses an SNP marker for detecting nasopharyngeal cancer susceptibility. The SNP marker comprises 21 SNP loci: rs9344, rs13347, rs1412829, rs9418990, rs915906, rs8192780, rs3827688, rs3813865, ra2249695, rs1536826, rs29232, rs2517713, rs2975042, rs3129055, rs3131866, rs22556218, rs10889677, rs6774494, rs243865, rs2285053 and rs9510787. The invention furthermore discloses a PCR amplification primer, a single-base extension primer and a kit of the SNP marker, so as to provide important basis for the illness risk assessment and diagnosis reference of nasopharyngeal cancer.
Owner:深圳市核子基因科技有限公司
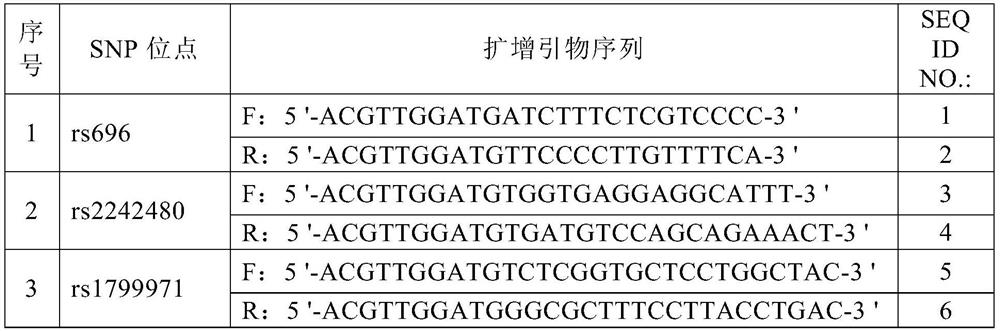
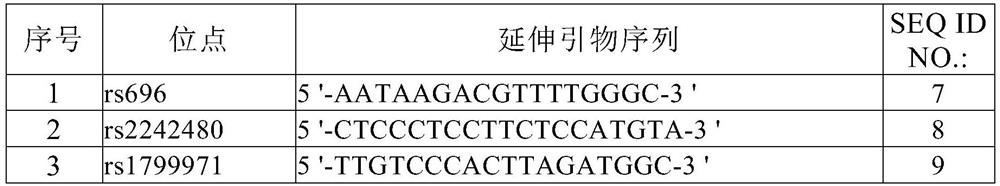
Specific molecular marker primers, kit and identification method for Thymallus arcticus grubei population
ActiveCN110042169AImprove accuracyGood value for moneyMicrobiological testing/measurementClimate change adaptationThymallus arcticus grubeiAgricultural science
The invention discloses specific molecular marker primers, a kit and an identification method for Thymallus arcticus grubei population. The primers are ThtC1010, ThtC1029 and ThtC1034, the sequence ofthe primer ThtC 1010 is shown in SEQ ID NO. 1-2, the sequence of the primer ThtC1029 is shown in SEQ ID NO. 3-4, and the sequence of the primer ThtC 1034 is shown in SEQ ID NO. 5-6. According to thepresent invention, the method is a new method based on the principle of molecular biology technology for distinguishing the Thymallus arcticus grubei population in China and other countries for the first time. EST-SSR markers are successfully developed on the basis of EST data of Thymallus arcticus grubei, the genetic background of the Thymallus arcticus grubei population is analyzed with partialmarkers, and specific markers capable of distinguishing three populations are found. Genhe graylling population can be accurately distinguished from the other two populations by a THC1010 locus and aTHC1029 locus, the three populations can be distinguished by a THC1034 locus, and the method has the advantages of high accuracy, good technical reproducibility and high cost performance.
Owner:HEILONGJIANG RIVER FISHERY RES INST CHINESE ACADEMY OF FISHERIES SCI
Kit for detecting ovarian cancer susceptibility and SNP (single nucleotide polymorphism) marker thereof
InactiveCN106434981AImprove detection success rateEarly Assessment ImplementationMicrobiological testing/measurementDNA/RNA fragmentationOvarian cancerBiology
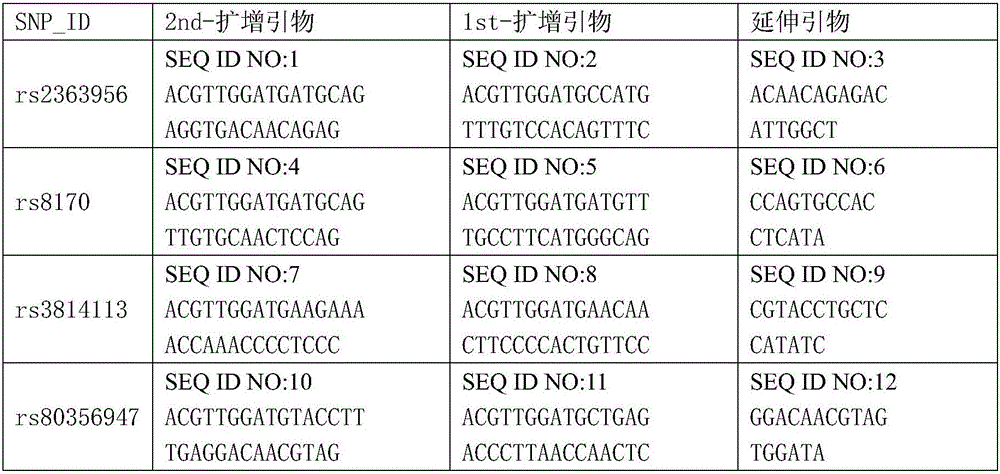
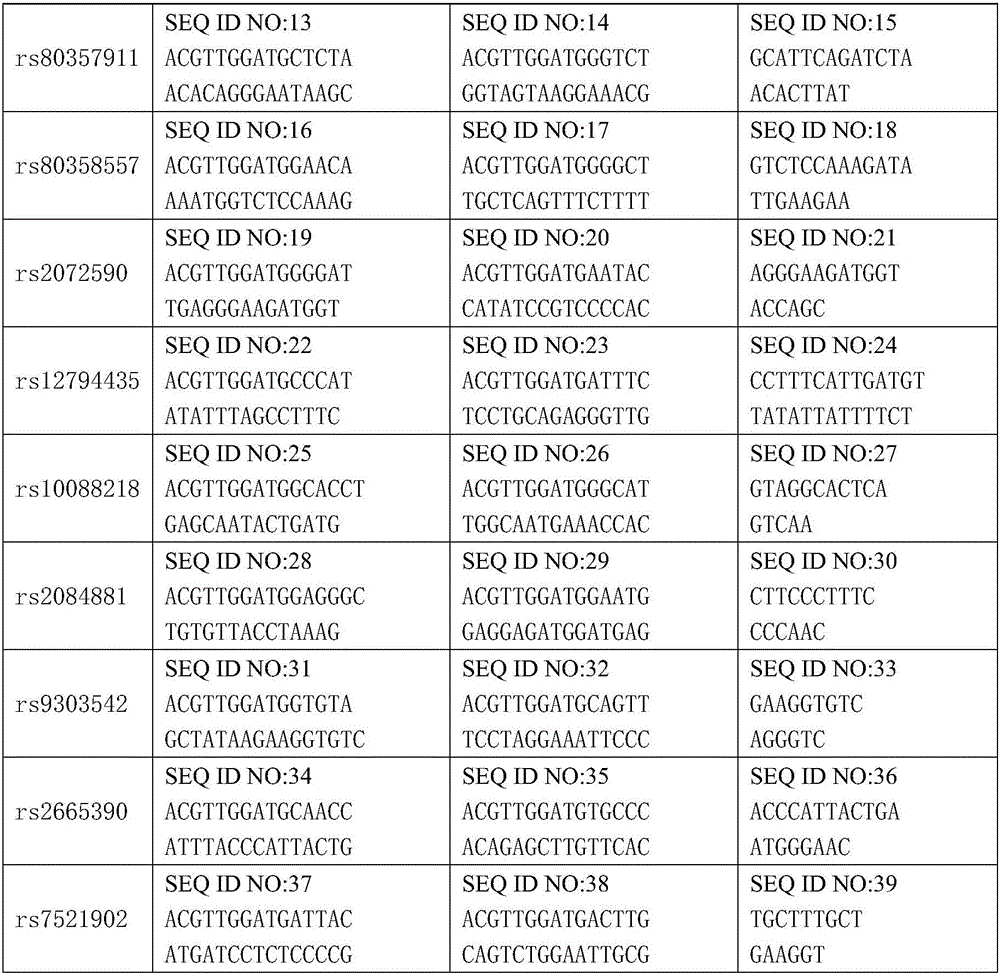
The invention discloses a kit for detecting ovarian cancer susceptibility, comprising 13 SNP sites, to be specific, rs2363956, rs8170, rs3814113, rs80356947, rs80357911, rs80358557, rs2072590, rs12794435, rs10088218, rs2084881, rs9303542, rs2665390 and rs7521902, and also discloses PCR (polymerase chain reaction) amplification primer and single-base extension primer of an SNP marker. The 13 SNP sites herein can provide important basis for the assessment and diagnosis of ovarian cancer risk.
Owner:深圳市核子基因科技有限公司
Kit for detecting esophagus cancer susceptivity and SNP (single nucleotide polymorphism) marker of kit
InactiveCN106434980AImprove detection success rateEarly Assessment ImplementationMicrobiological testing/measurementDNA/RNA fragmentationGeneticsEsophagus Cancers
The invention discloses an SNP (single nucleotide polymorphism) marker for detecting esophagus cancer susceptivity. The SNP marker comprises 24 SNP loci. The invention further discloses a PCR (polymerase chain reaction) amplification primer, a single-basic-group extension primer and a kit of the SNP marker. An important basis is provided for illness risk evaluation and diagnosis reference of esophagus cancer.
Owner:深圳市核子基因科技有限公司
Early screening method and kit of ankylosing spondylitis susceptibility gene
ActiveCN113025702AImprove detection success rateGood technical reproducibilityMicrobiological testing/measurementDNA/RNA fragmentationSpondarthritisMass analyzer
The invention provides an early screening method of ankylosing spondylitis susceptibility genes and a kit, and particularly, the invention takes differences between gene spectrums of Chinese people and European and American people into consideration, and screens out a combination of SNP loci of ankylosing spondylitis susceptibility related genes of Chinese people; a nucleic acid mass spectrometer is used for wide (high-throughput detection sites and high-throughput detection samples) screening and inspection of genetic markers related to ankylosing spondylitis. The method disclosed by the invention is high in detection success rate, good in technical reproducibility and high in cost performance, can realize multi-gene detection of a single small sample, and meets maximum use of the small sample; the method has the technical advantages of high accuracy and high sensitivity, the detection result is stable, and the detection positive rate is increased.
Owner:北京科力丹迪生物医疗科技有限公司
Method and kit for guiding carbamazepine individualized medication genes
PendingCN112501283AImprove detection success rateGood technical reproducibilityMicrobiological testing/measurementDNA/RNA fragmentationMass analyzerCarbamazepine
The invention provides a method and kit for guiding carbamazepine individualized medication genes. Particularly, a combination of SNP loci of genes related to carbamazepine individualized medication is screened out by considering the difference of medication curative effects of different epileptic patients, and genetic markers related to carbamazepine are widely screened and inspected (with high-throughput detection sites and high-throughput detection samples) by utilizing a nucleic acid mass spectrometer. The method disclosed by the invention is high in detection success rate, good in technical reproducibility and high in cost performance, can realize detection of multiple genes of a single small sample, and meets the maximized use demand of the small sample; and the method provided by the invention has the technical advantages of high accuracy and high sensitivity, the detection result is stable, and the detection positive rate is increased.
Owner:广东南芯医疗科技有限公司
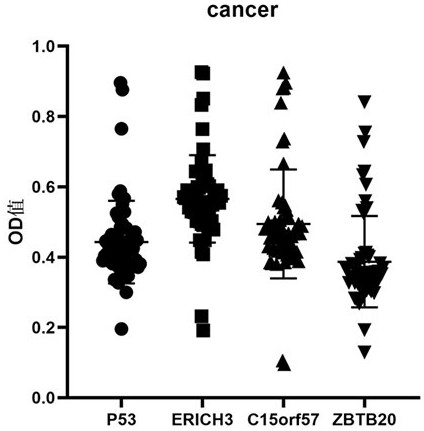
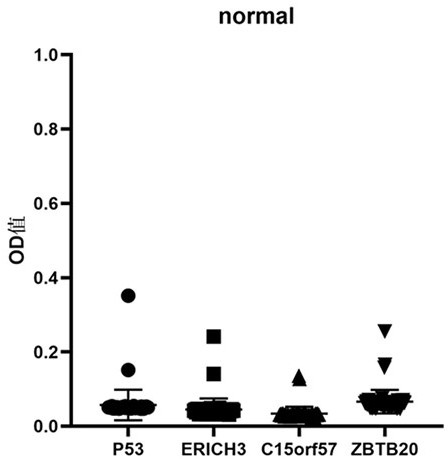
Early esophageal squamous cell carcinoma screening kit based on group of tumor-associated antigens
ActiveCN113075413AEfficient detectionHigh detection sensitivityBiological testingElisa kitP53 protein
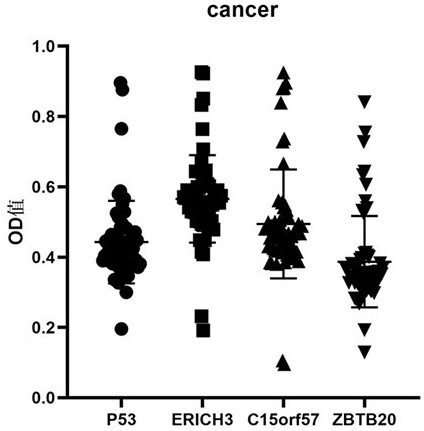
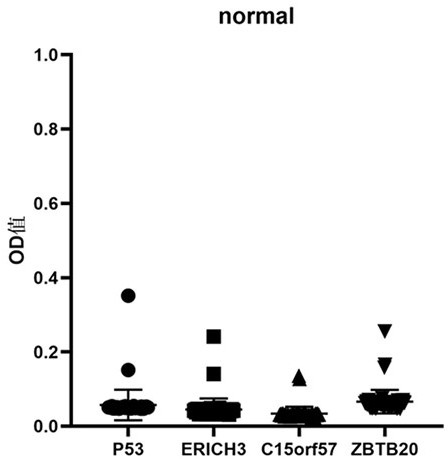
The invention belongs to the technical field of medical biology, and particularly discloses an early esophageal squamous cell carcinoma screening kit based on a group of tumor-associated antigens, the kit comprises a solid-phase carrier and the tumor-associated antigens coated on the solid-phase carrier, and the tumor-associated antigens are composed of P53 protein, ZBTB20 protein, ERICH3 protein and C15orf57 protein. Furthermore, the kit also comprises a sample diluent, a second antibody, a second antibody diluent, positive control serum, negative control serum, a developing solution, a stop solution and a washing solution. The ELISA kit can effectively detect esophageal cancer, especially early esophageal cancer, the detection sensitivity of the ELISA kit reaches up to 82%, the specificity reaches 84%, and the ELISA kit can be used for large-scale screening of asymptomatic crowds in esophageal cancer high-incidence areas and is beneficial to screening and early discovery of asymptomatic high-risk crowds.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
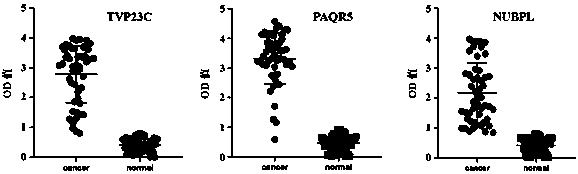
Liquid biopsy ELISA kit for early screening of esophageal cancer high-risk groups
ActiveCN111551545AEfficient detectionHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorBiological testingElisa kitAntiendomysial antibodies
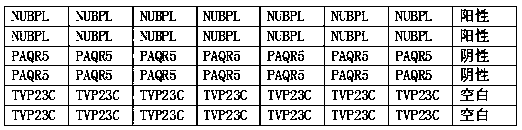
The invention belongs to the technical field of bioengineering and biomedicine, particularly belongs to the field of molecular biology and oncology, and more particularly relates to a liquid biopsy ELISA kit for early screening of esophageal cancer high-risk groups. The kit comprises a solid-phase carrier and tumor-associated antigens TVP23C, PAQR5 and NUBPL coated on the solid-phase carrier, andfurther comprises a sample diluent, a second antibody, a second antibody diluent, negative control serum, positive control serum, a washing solution, a developing solution and a stop solution. The kithas esophageal cancer detection sensitivity of 91.46% and esophageal cancer detection specificity of 83.75%, and is suitable for large-scale screening of asymptomatic people in esophageal cancer high-incidence areas.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Method and kit for guiding epirubicin individualized medication gene
PendingCN112695094AImprove detection success rateGood technical reproducibilityMicrobiological testing/measurementDNA/RNA fragmentationMedicineMass analyzer
The invention provides a method and kit for guiding an epirubicin individualized medication gene, and particularly relates to a method for screening out a combination of SNP loci of genes related to epirubicin individualized medication by considering the difference of medication curative effects of different cancer patients. A nucleic acid mass spectrometer is used for widely screening and inspecting epirubicin-related genetic markers (high-throughput detection sites and high-throughput detection samples). The method disclosed by the invention is high in detection success rate, good in technical reproducibility and high in cost performance, can realize detection of multiple genes of a single small sample, and meets maximized use of the small sample; and the method provided by the invention has the technical advantages of high accuracy and high sensitivity, the detection result is stable, and the detection positive rate is improved.
Owner:广东南芯医疗科技有限公司
Screening kit for early esophageal squamous cell carcinoma based on a set of tumor-associated antigens
ActiveCN113075413BEfficient detectionHigh detection sensitivityBiological testingElisa kitP53 protein
The invention belongs to the field of medical biotechnology, and specifically discloses an early esophageal squamous cell carcinoma screening kit based on a group of tumor-associated antigens. The kit includes a solid-phase carrier and a tumor-associated antigen coated on the solid-phase carrier. The tumor-associated antigens are composed of P53 protein, ZBTB20 protein, ERICH3 protein and C15orf57 protein. Further, the kit also includes sample diluent, secondary antibody, secondary antibody diluent, positive control serum, negative control serum, chromogenic solution, stop solution and washing solution. The ELISA kit of the present invention can effectively detect esophageal cancer, especially early-stage esophageal cancer, with a detection sensitivity of up to 82% and a specificity of 84%, and can be used for large-scale screening of asymptomatic people in high-incidence areas of esophageal cancer, which is beneficial to asymptomatic Screening and early detection of people at high risk of symptoms.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Momordica charantia powder containing beta-cyclodextrin and preparation method of momordica charantia powder
PendingCN111569087AEasy to prepareShorten the production cycleMetabolism disorderPharmaceutical non-active ingredientsMomordicaCyclodextrin
The invention discloses momordica charantia powder containing beta-cyclodextrin and a preparation method of the momordica charantia powder. The momordica charantia powder containing beta-cyclodextrinis prepared by mixing and crushing momordica charantia coarse particles and beta-cyclodextrin and then carrying out inclusion, wherein the mass ratio of momordica charantia to beta-cyclodextrin is 1:(0.1-2.0). Compared with the prior art, the invention provides a novel momordica charantia processing technology and a related product, and the preparation method is simple and convenient, short in production period, good in technical reproducibility and high in stability; and the obtained product has the advantages of strong solubility, good stability, good cyclodextrin inclusion effect of the powder, fast dissolution and good quality.
Owner:江苏天美健大自然生物工程有限公司
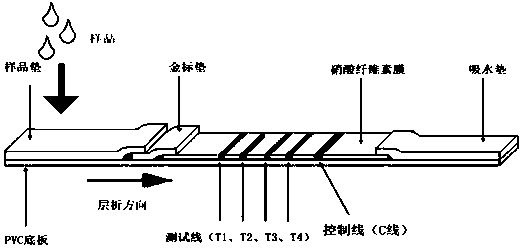
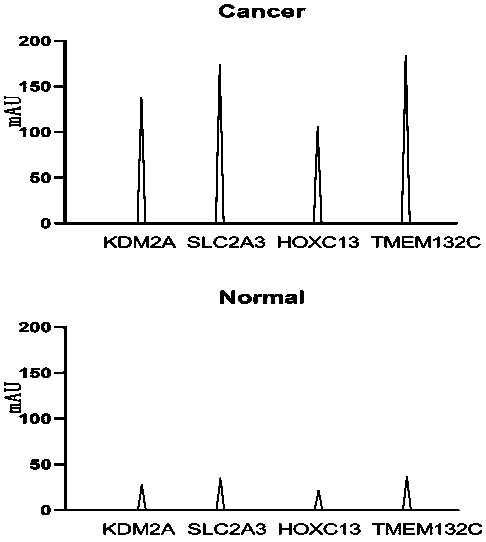
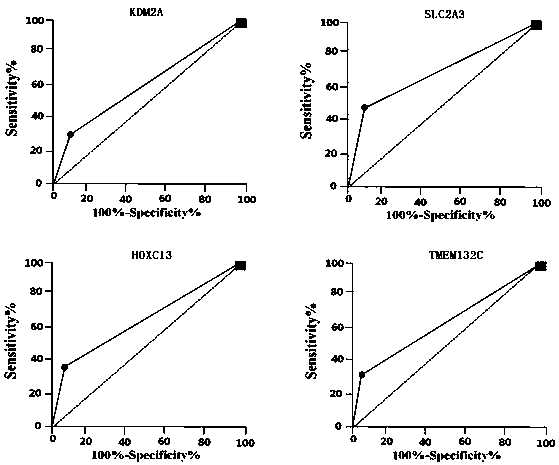
Group of multi-index combined detection colloidal gold test strips for early esophageal cancer screening
ActiveCN111562379AHigh detection sensitivityConducive to screeningDisease diagnosisAntigenSpecific detection
The invention belongs to the technical field of bioengineering and biomedicines, particularly relates to the field of oncology, and more particularly relates to a group of multi-index combined detection colloidal gold test strips for early esophageal cancer screening. According to the colloidal gold test strips, specific detection reagents of four antigens, namely KDM2A, SLC2A3, HOXC13 and TMEM132C, are combined to detect the early esophageal cancer; the detection sensitivity is as high as 93.7%, and the specificity reaches 71.4%, that is, the colloidal gold test strips provided by the invention have high sensitivity and specificity for early esophageal cancer detection, and are suitable for large-scale screening of asymptomatic people in esophageal cancer high-incidence areas.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
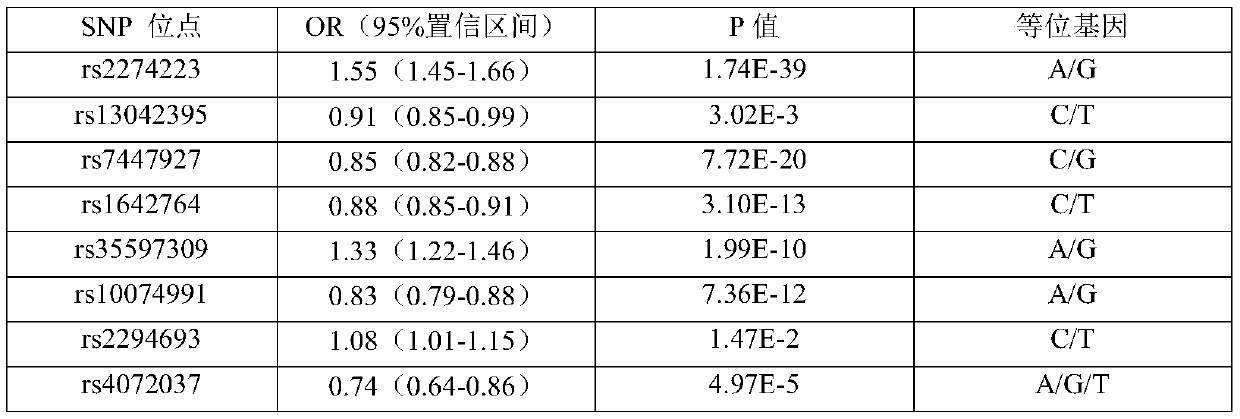
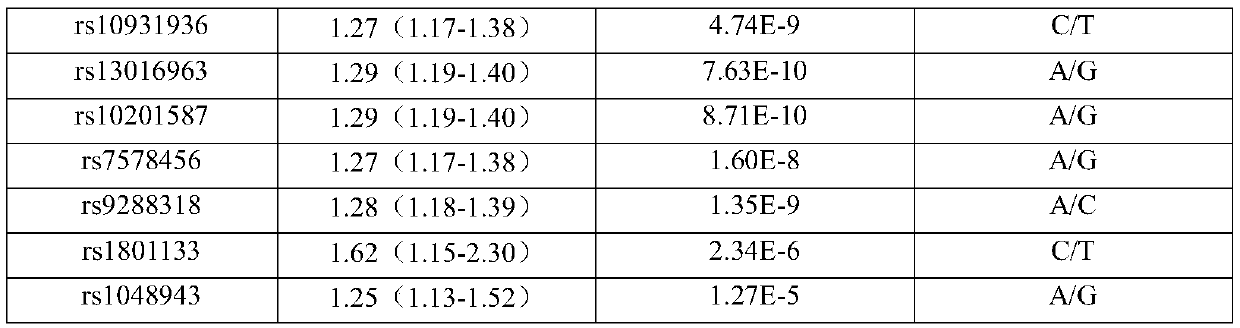
A SNP marker and kit for screening high-risk groups of cardiac cancer
ActiveCN110343765BImprove detection success rateGood technical reproducibilityMicrobiological testing/measurementDNA/RNA fragmentationGastric Cardia CarcinomaOncology
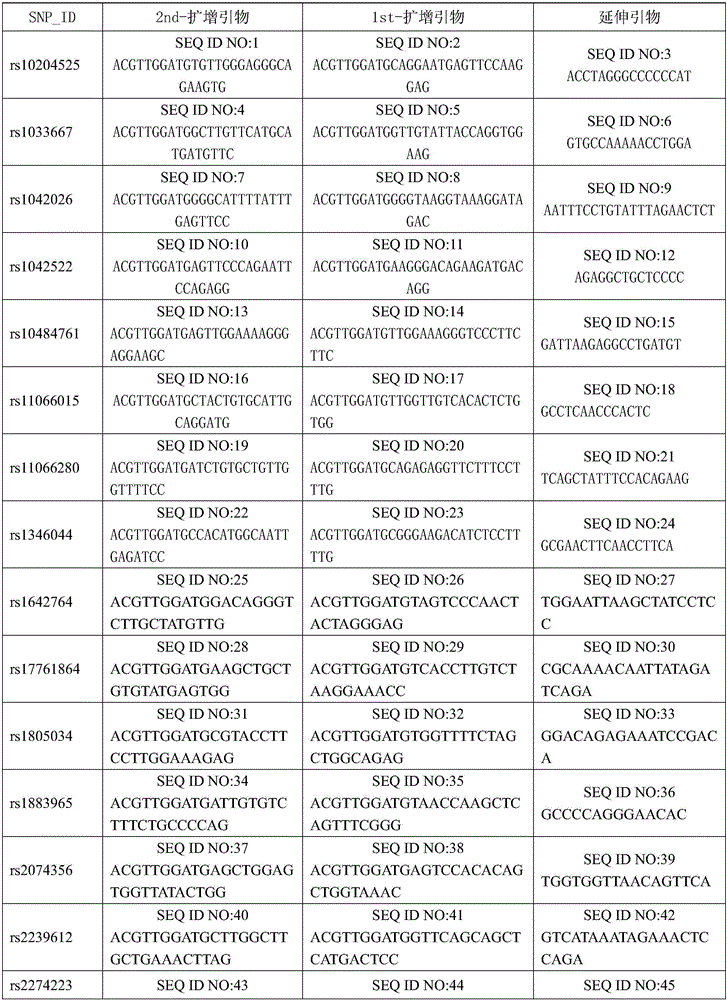
The invention belongs to the field of genetic engineering and tumor medicine, and particularly discloses an SNP marker and kit for screening out and diagnosing a gastric cancer high-risk group. The SNP marker is a combination of rs2274223, rs13042395 rs7447927, rs1642764 rs35597309, rs10074991, rs2294693, rs4072037, rs10931936, rs13016963, rs10201587, rs7578456, rs9288318, rs1801133 and rs1048943.The invention further discloses a kit for screening out and the gastric cancer high-risk group. The SNP marker combination can be used for early evaluating and large-scale screening of the gastric cancer, is high in detection success rate and technical reproducibility, defines the gastric cancer high-risk group to the maximum extent, makes the early gastric cancer detection rate reach 71.4%, andprovides an important basis for screening out the gastric cancer high-risk group and evaluating the disease risk.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Guiding method and kit for sufentanil individualized medication genes
PendingCN112553325AImprove detection success rateGood technical reproducibilityMicrobiological testing/measurementMedication doseMedicine
The invention provides a guiding method and kit for sufentanil individualized medication genes. Particularly, the method considers differences of medication doses of different patients, screens out acombination of SNP loci of sufentanil individualized medication related gene, and uses a nucleic acid mass spectrometer for widely screening and inspecting sufentanil-related genetic markers (in a manner of high-throughput detection sites and high-throughput detection samples). The method has a high detection success rate, good technical reproducibility and high cost performance, can realize detection of multiple genes of a single small sample, and meets maximized use of the small sample. The method has technical advantages of high accuracy and high sensitivity, and a stable detection result,and improves a detection positive rate.
Owner:广东南芯医疗科技有限公司
Early screening method and kit for Graves' disease (GD) susceptibility genes
PendingCN112592972AImprove detection success rateGood technical reproducibilityMicrobiological testing/measurementDNA/RNA fragmentationGenetic markerSusceptibility gene
The invention provides an early screening method and kit for Graves' disease (GD) susceptibility genes. Particularly, a combination of SNPsites of genes related to GD susceptibility of Chinese peopleis screened by considering the difference of gene profiles of Chinese people and European and American people, a nucleic acid mass spectrometer is used for widely screening and inspecting genetic markers (high-throughput detection sites and high-throughput detection samples) related to GD. The method is high in detection success rate, good in technical reproducibility and high in cost performance,can realize detection of multiple genes of a single small sample, and meets maximized use of the small sample; and the method has the technical advantages of high accuracy and high sensitivity, the detection result is stable, and the detection positive rate is improved.
Owner:广东南芯医疗科技有限公司
Application of a group of tumor-associated antigens in the preparation of early screening kits for cardia cancer
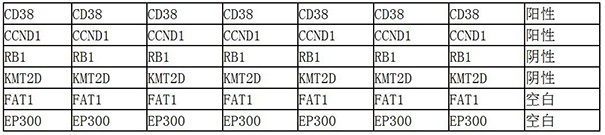
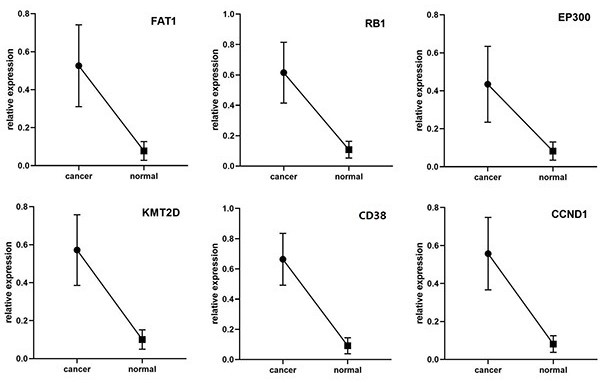
The invention belongs to the field of molecular biology and oncology, and particularly relates to an application of a group of tumor-associated antigens in preparation of a cardia cancer early screening kit. The invention discloses a combination of tumor-associated antigens CD38, CCND1, RB1, KMT2D, FAT1 and EP300 for preparing a cardia cancer early screening kit. The ELISA kit for screening the early cardia cancer comprises a solid-phase carrier and tumor-associated antigens coating the solid-phase carrier, and the tumor-associated antigens are CD38, CCND1, RB1, KMT2D, FAT1 and EP300. According to the invention, the six tumor-associated antigens including CD38, CCND1, RB1, KMT2D, FAT1 and EP300 are taken as a combination for the first time, the combination is used for detecting the cardiacancer, especially the early cardia cancer, and the kit has the detection sensitivity of 94.74% and the specificity of 84.21%.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
An autoantibody combined detection ELISA kit for early screening of esophageal squamous cell carcinoma
ActiveCN110187113BEfficient detectionReduce mortalityMaterial analysisParanasal Sinus CarcinomaAutoantibody
The invention belongs to the technical field of medical oncology, and particularly discloses an autoantibody joint detection ELISA kit for early screening of esophageal squamous carcinoma. The kit comprises a solid-phase carrier and a tumor-associated antigen coated on the solid-phase carrier, wherein the tumor-associated antigen consists of P53, TP53, CDKN2B and NPM1. Furthermore, the kit comprises sample diluent, a second antibody, second antibody diluent, positive control serum, negative control serum, developing solution, stop solution and washing solution. The ELISA kit is capable of effectively detecting the esophageal squamous carcinoma, especially the early esophageal squamous carcinoma, has detection sensitivity up to 88.8% and specificity up to 86.3%, can be used for the large-scale screening of asymptomatic groups in high-risk areas of the esophageal squamous carcinoma, and is beneficial for the esophageal squamous carcinoma screening and early discovery of the asymptomatichigh-risk groups.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Guiding method and kit for atorvastatin individualized medication gene
ActiveCN112646869AImprove detection success rateGood technical reproducibilityMicrobiological testing/measurementAgainst vector-borne diseasesMedicineMass analyzer
The invention provides a guiding method and a kit for an atorvastatin individualized medication gene, and in particular relates to a combination of SNP sites of an atorvastatin individualized medication related gene. A nucleic acid mass spectrometer is used for widely screening and inspecting atorvastatin-related genetic markers (high-throughput detection sites and high-throughput detection samples). The method is high in detection success rate, good in technical reproducibility and high in cost performance, can realize detection of multiple genes of a single small sample, and meets maximized use of the small sample; and the method provided has the technical advantages of high accuracy and high sensitivity, the detection result is stable, and the detection positive rate is improved.
Owner:广东南芯医疗科技有限公司
An ELISA kit for early diagnosis of esophageal squamous cell carcinoma
The invention belongs to the technical field of medical biology, and particularly discloses an ELISA kit for early diagnosis of esophageal squamous cell carcinoma. The kit comprises a solid phase carrier and a tumor-associated antigen coated on the solid phase carrier, and the tumor-associated antigen is composed of HMGB1, TLR4, TAK1, ATG5, SLC22A3 and CD38. Furthermore, the kit also comprises a sample diluent, a second antibody, a second antibody diluent, positive control serum, negative control serum, a developing solution, a stop solution and a washing solution. The ELISA kit provided by the invention can effectively detect esophageal squamous cell carcinoma, especially early esophageal squamous cell carcinoma, has detection sensitivity as high as 92.7% and specificity as high as 84.0%,can be used for large-scale screening and diagnosis of asymptomatic people in esophageal squamous cell carcinoma high-incidence areas, and is beneficial to screening and early discovery of asymptomatic high-risk people.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
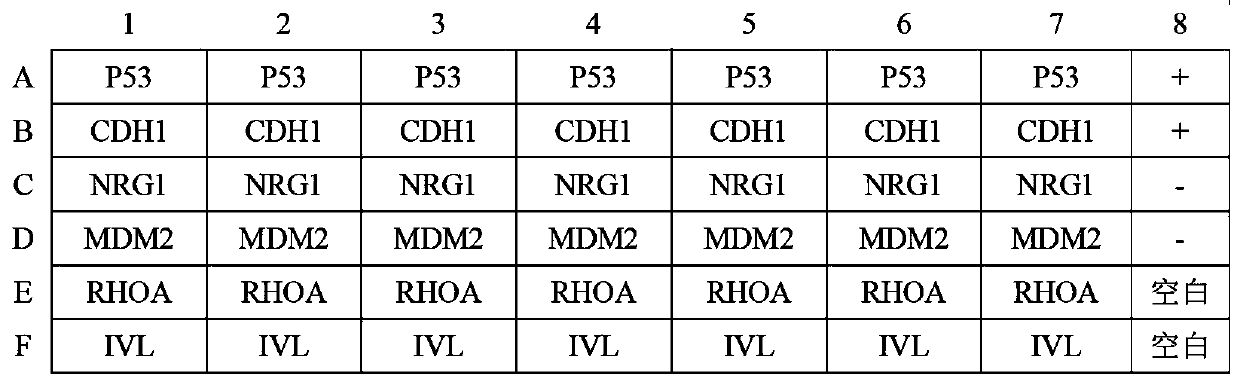
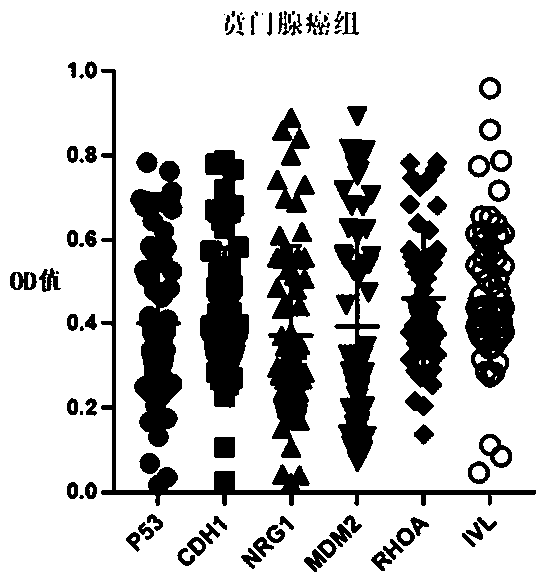
Autoantibody joint detection ELISA kit for early cardia adenocarcinoma screening
ActiveCN111323587AEfficient detectionHigh detection sensitivityMaterial analysisAutoantibodyElisa kit
The invention belongs to the technical field of medical biology, and specifically discloses an autoantibody joint detection ELISA kit for early cardia adenocarcinoma screening. The kit comprises a solid phase carrier and a tumor-associated antigen coated on the solid phase carrier, and the tumor-associated antigen is composed of P53, CDH1, NRG1, MDM2, RHOA and IVL. Furthermore, the kit also comprises a sample diluent, a second antibody, a second antibody diluent, positive control serum, negative control serum, a developing solution, a stop solution and a washing solution. The ELISA kit provided by the invention can effectively detect cardia adenocarcinoma, especially early cardia adenocarcinoma, has detection sensitivity as high as 86% and specificity as high as 88%, can be used for large-scale screening of asymptomatic people in cardia adenocarcinoma high-incidence areas, and is beneficial to screening and early discovery of asymptomatic high-risk people.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
An ELISA kit for screening early cardia cancer
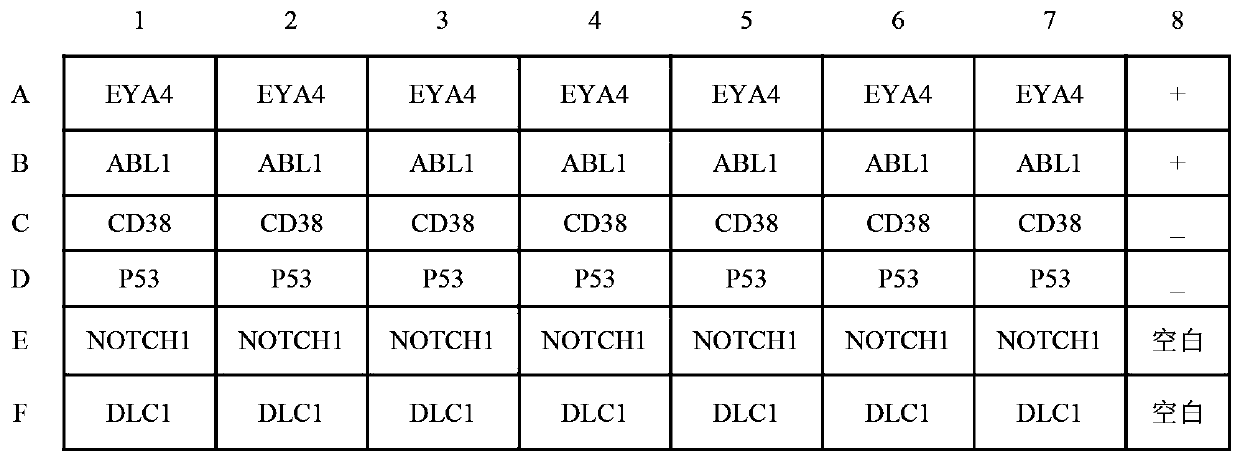
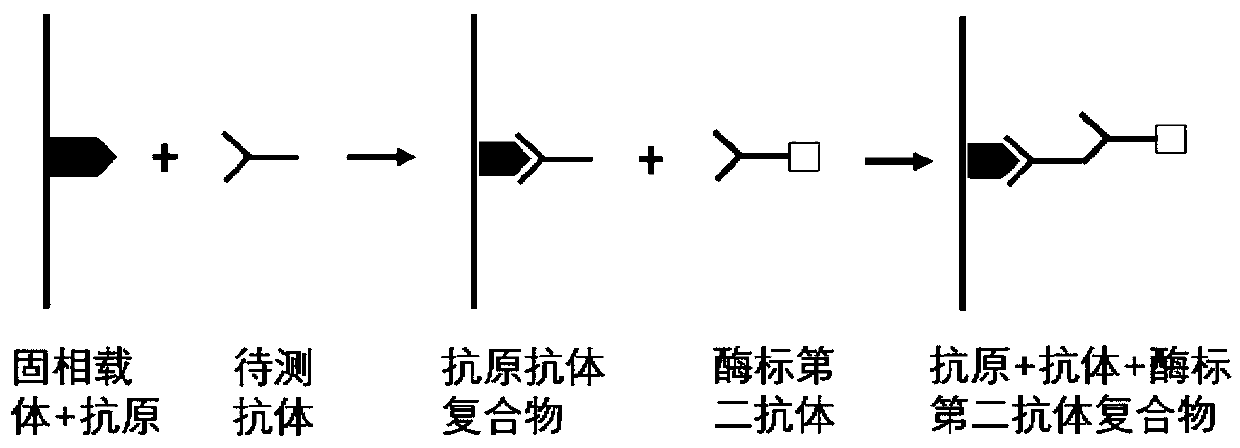
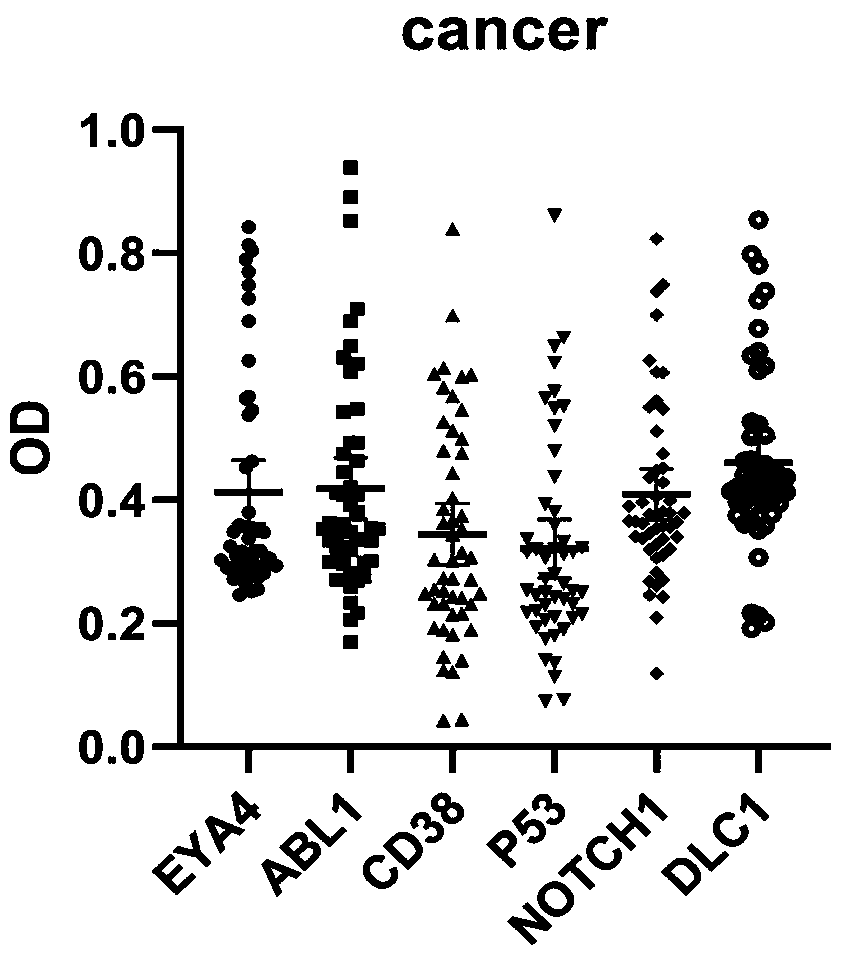
ActiveCN110187111BEfficient detectionHigh detection sensitivityMaterial analysisElisa kitGastric Cardia Carcinoma
The invention belongs to the technical field of tumor medicine, and particularly discloses an ELISA kit for screening of early cardia cancer. The kit comprises a solid phase carrier and a tumor-associated antigen coating the solid phase carrier, wherein the tumor-associated antigen is formed by EYA4, ABL1, CD38, P53, NOTCH1 and DLC1. Furthermore, the kit further comprises a sample diluent, a second antibody, a second antibody diluent, positive control serum, negative control serum, a developing solution, a stop solution and a washing solution. According to the ELISA kit disclosed by the invention, the cardia cancer, especially the early cardia cancer, can be effectively detected, the detection sensitivity is up to 92.2%, the specificity is up to 79.6%, the ELISA kit can be used for large-scale screening of an asymptomatic population in a high-incidence area of the cardia cancer, and is beneficial to screening and early discovery of the cardia cancer of the asymptomatic high-risk population.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com