Screening kit for early esophageal squamous cell carcinoma based on a set of tumor-associated antigens
A technology of tumor-associated antigen and esophageal squamous cell carcinoma, which is applied in the field of medicine and biology, can solve the problems of no screening of esophageal squamous cell carcinoma, the detection rate of esophageal cancer is less than 2%, and the mortality rate has not been significantly improved, so as to ensure detection sensitivity and avoid Physical and mental pain, strong balance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
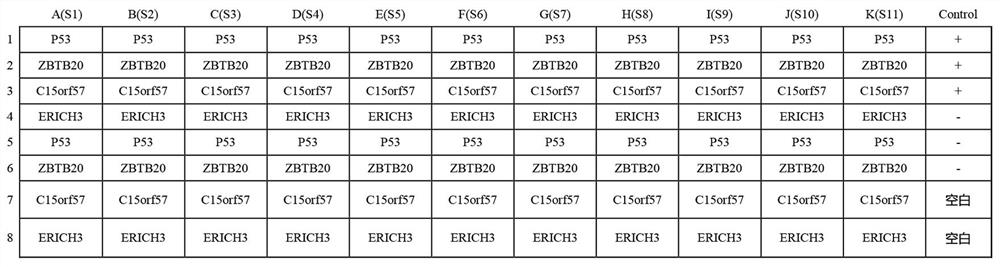
[0039] Embodiment 1: the preparation of kit
[0040] According to the principle of indirect ELISA, the invention prepares an autoantibody combined detection ELISA kit that can be used for screening and diagnosing early esophageal squamous cell carcinoma. The principle of indirect enzyme-linked immunoassay is to connect the antigen to a solid-phase carrier, and the antibody to be tested in the sample combines with it to form a solid-phase antigen-test antibody complex, and then use the enzyme-labeled secondary antibody and the solid-phase antigen-test antibody to The antibodies in the complex combine to form a solid-phase antigen-test antibody-enzyme-labeled secondary antibody complex, and then measure the degree of color development after adding the substrate to determine the content of the antibody to be tested.
[0041] 1. Experimental materials and reagents:
[0042] (1) Four kinds of tumor-associated antigen proteins (P53, ZBTB20, ERICH3 and C15orf57), purchased from Wuha...
Embodiment 2
[0076] Embodiment 2: the usage method of kit
[0077] 1. Serum sample incubation:
[0078] Dilute the serum sample to be tested with the sample diluent at a ratio of 1:500, and then add the diluted serum sample to the reaction wells of the 96-well microplate plate coated with antigen, with a sample volume of 100 μl / well, Place in a constant temperature incubator at 37°C and incubate for 1 h, then discard the liquid in the reaction well, and wash with washing solution 3 times, each time for 3 min.
[0079] 2. Enzyme-labeled secondary antibody incubation:
[0080] Dilute the horseradish peroxidase-labeled RecA protein with the secondary antibody diluent at a ratio of 1:40000, and then add the diluted horseradish peroxidase-labeled RecA protein to the reaction wells of the 96-well microtiter plate In this method, the sample volume was 100 μl / well, placed in a 37°C constant temperature incubator and incubated for 50 minutes, then the liquid in the sample wells was discarded, and...
Embodiment 3
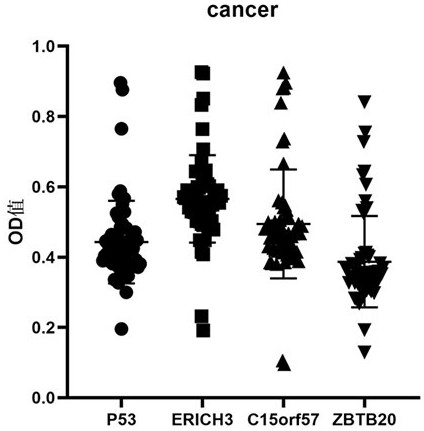
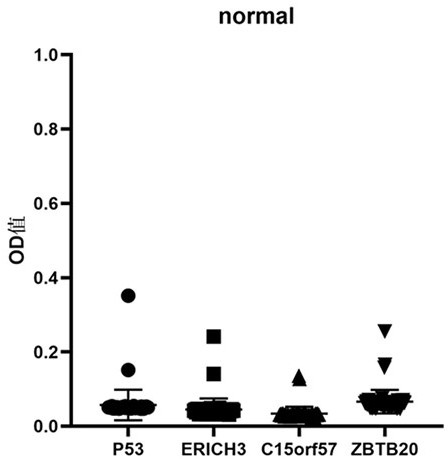
[0085] Embodiment 3: Analysis of the diagnostic value of the kit of the present invention
[0086] In this example, the kit prepared in Example 1 is used to detect the serum samples of patients with early esophageal squamous cell carcinoma and normal people according to the method described in Example 2, so as to evaluate and analyze the kit of the present invention for screening and screening of early esophageal squamous cell carcinoma. diagnostic value.
[0087] 1. Sample source
[0088] 200 serum samples were collected from the State Key Laboratory of Esophageal Cancer Prevention and Control in the First Affiliated Hospital of Zhengzhou University, including 100 samples of normal human serum (control group) and 100 serum samples of patients with early esophageal squamous cell carcinoma (esophageal squamous cell carcinoma group). 100 cases of normal human serum were collected from the healthy physical examination center of the laboratory's cooperative hospital, without any ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com