Autoantibody joint detection ELISA kit for early cardia adenocarcinoma screening
A technology of combined detection of autoantibodies, applied in the fields of molecular biology and oncology, to achieve strong balance, reduce misdiagnosis rate, and improve specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of tumor-associated antigens
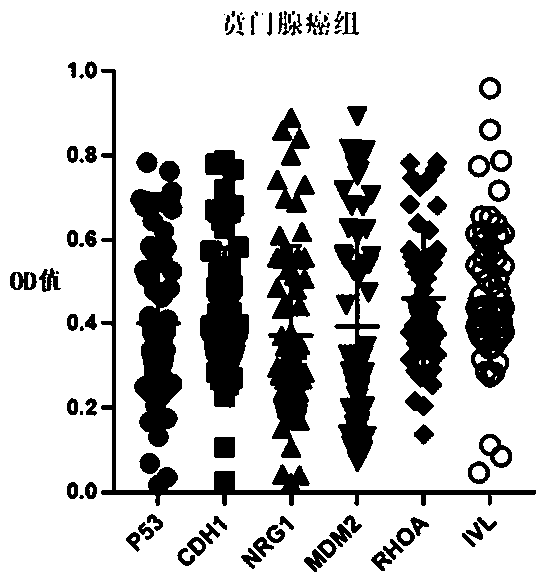
[0030] By using a prokaryotic expression system, six tumor-associated antigens (P53, CDH1, NRG1, MDM2, RHOA, and IVL) were prokaryotically expressed and purified in order to prepare for the next experiment. The specific antigen preparation process is as follows:
[0031] (1) Using gene cloning technology to construct recombinant prokaryotic expression plasmids of six tumor-associated antigen proteins (P53, CDH1, NRG1, MDM2, RHOA and IVL);
[0032] (2) Expressing the target protein: Transform the constructed recombinant prokaryotic expression plasmids into Escherichia coli BL21(DE3) and use IPTG (isopropylthiogalactopyranoside) to induce the expression of the target protein;
[0033] (3) Purify the target protein: according to the tag carried by the target protein, use the traditional corresponding purification scheme to purify the target protein;
[0034] (4) The protein concentration was measured by Bradford metho...
Embodiment 2
[0036] Embodiment 2: the preparation of kit
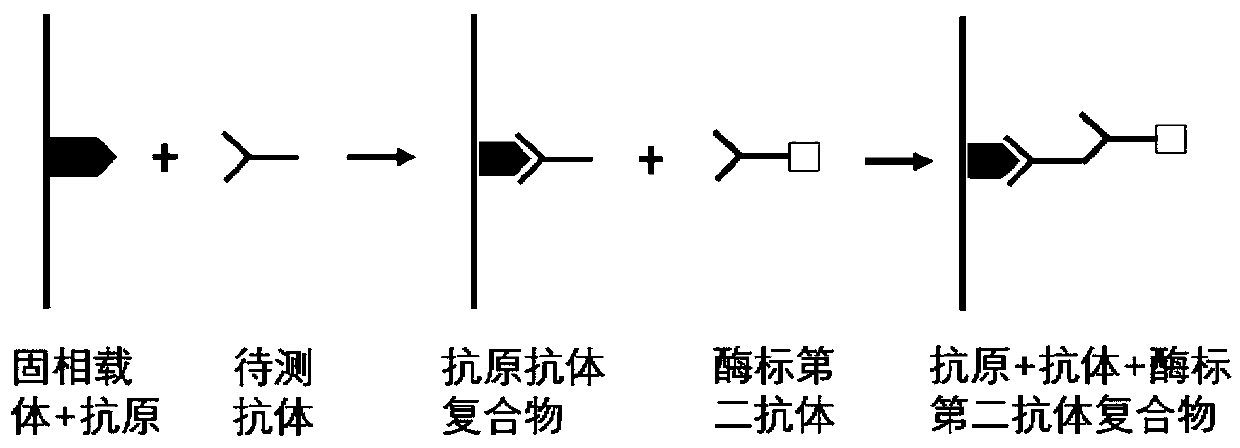
[0037] According to the principle of indirect ELISA, the invention prepares an autoantibody combined detection ELISA kit for screening and diagnosing early cardia adenocarcinoma. The principle of indirect enzyme-linked immunoassay is to connect the antigen to a solid-phase carrier, and the antibody to be tested in the sample combines with it to form a solid-phase antigen-test antibody complex, and then use the enzyme-labeled secondary antibody and the solid-phase antigen-test antibody to The antibody in the complex is combined to form a solid-phase antigen-test antibody-enzyme-labeled secondary antibody complex, and then measure the degree of color development after adding the substrate to determine the content of the antibody to be tested (see figure 2 ).
[0038] 1. Experimental materials and reagents:
[0039] (1) 6 tumor-associated antigen proteins (P53, CDH1, NRG1, MDM2, RHOA and IVL);
[0040] (2) 48-well ELISA plate: pur...
Embodiment 3
[0073] Embodiment 3: the usage method of kit
[0074] 1. Serum sample incubation:
[0075] Dilute the serum sample to be tested with the sample diluent at a ratio of 1:100, and then add the diluted serum sample into the reaction wells of the 48-well microplate plate coated with antigen, with a sample volume of 100 μl / well, Place in a constant temperature incubator at 37°C and incubate for 1 h, then discard the liquid in the reaction well, and wash with washing solution 5 times, each time for 3 min.
[0076] 2. Enzyme-labeled secondary antibody incubation:
[0077] Dilute the horseradish peroxidase-labeled RecA protein with the secondary antibody diluent at a ratio of 1:80000, and then add the diluted horseradish peroxidase-labeled RecA protein to the reaction wells of the 48-well microtiter plate In this method, the sample volume was 100 μl / well, and incubated in a constant temperature incubator at 37°C for 1 hour, then the liquid in the sample well was discarded, and washed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com