Method for directional differentiation of induced pluripotent stem cells into lymphoid tissue induced cells
A technology of pluripotent stem cells and lymphoid tissue, applied in the field of induced pluripotent stem cells to differentiate into lymphoid tissue induced cells, can solve the problems of low reproducibility, low differentiation efficiency, long cycle, etc., and achieve highly reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
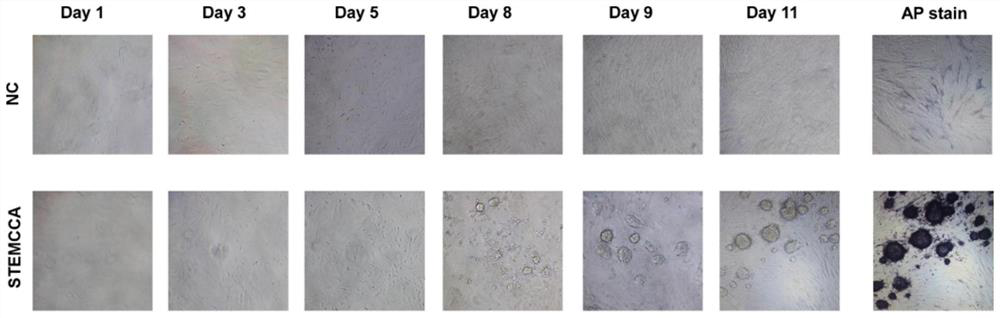
[0037] Embodiment 1, mouse embryonic fibroblast (MEF) preparation induced pluripotent stem cell (iPS)
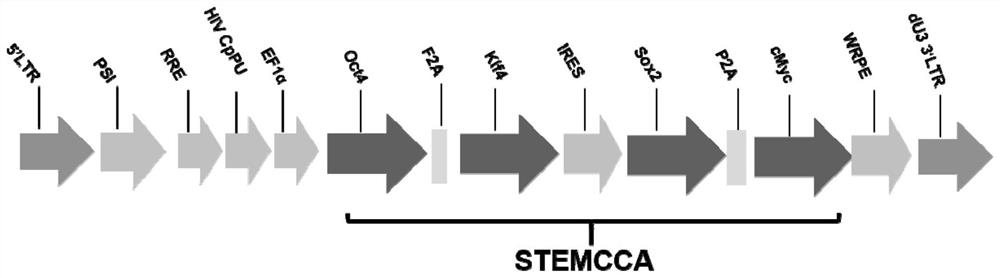
[0038] 1) Take C57 / BL6 mice aged 5-6 weeks, and put them together at 18:00 on the same day with a male-to-female ratio of 2:1. Check the mating situation of the female mice the next morning. The white vaginal plug in the vagina of the female mice is the sign of pregnancy. They were reared in separate cages and recorded as 0.5 days pregnant. Take the C57 / BL6 mouse embryos on the 13.5th day of pregnancy, remove the head / viscera and limbs of the embryos, add an appropriate amount of 0.25% trypsin-EDTA to digest and prepare mouse embryonic fibroblasts (MEFs), wash and centrifuge with 10 The complete culture medium of %FBS was seeded into T75 culture flasks and cultured in a 5% CO2 incubator at 37°C. After the cells grew to 85-90% confluence, they could be digested and passaged. The MEFs within the third generation were planted at a suitable density, and the lentivirus construct...
Embodiment 2
[0045] Example 2, Construction of Induced Pluripotent Stem Cells Overexpressed by Transcription Factor Combinations
[0046] The monoclonal induced pluripotent stem cells identified by pluripotency were expanded and cultured, and the induced pluripotent stem cells in the logarithmic growth phase were taken, digested and counted, seeded in a well plate at 5x104 power / ml, and mixed with MOI=100 Induced pluripotent stem cells were infected with transcription factor lentivirus, and the overexpression of exogenous TF gene was verified by q-PCR. The sequences of primers used to detect transcription factors are shown in Table 1.
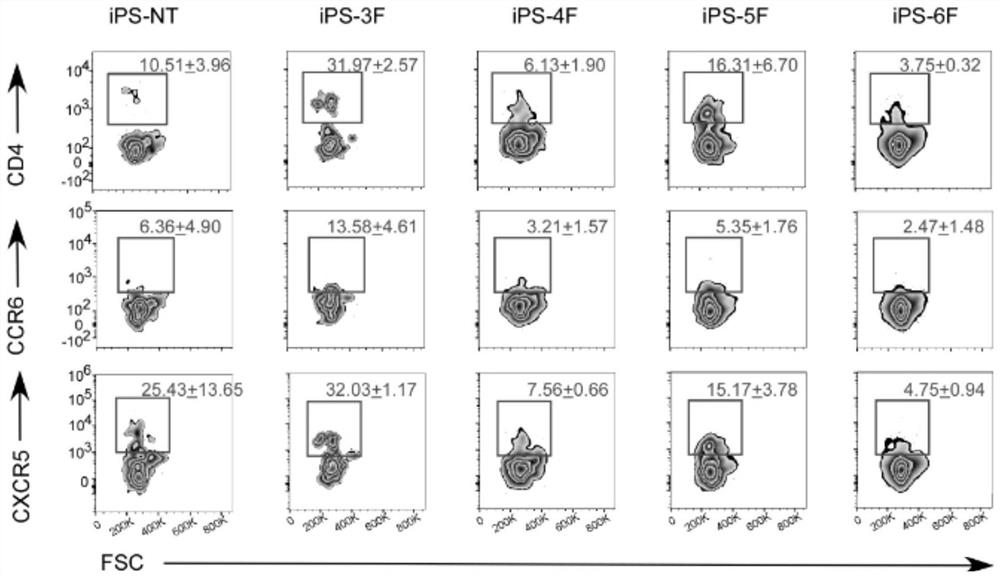
[0047] The inventor selected six transcription factors (transcription factor Runx1, transcription factor Tcf1, transcription factor Id2, transcription factor Rorγt, transcription factor Batf3 and transcription factor Nfil3), and found that these six transcription factors were overexpressed in the induced pluripotent stem cells. After expression, the induce...
Embodiment 3
[0055] Example 3. Differentiation of induced pluripotent stem cells overexpressed in combination of transcription factors into lymphoid tissue induced cells
[0056] (1) Digest and resuspend the cells in the first differentiation medium (15% fetal bovine serum + IMEM + 1% glutamine + 50ug / ml ascorbic acid + 5ng / ml bone morphogenetic protein 4) to a cell density of 1x10 5 / ml, 20ul / drop was cultured for 2.5 days by hanging drop culture method to prepare embryoid bodies (embryonic body, EB).
[0057] (2) EBs of D2.5 were collected and transferred to well plates pre-coated with 0.1% gelatin. In the second differentiation medium (15% FBS + IMEM + 1% glutamine + 50 ug / ml ascorbic acid + 5 ng / ml bone morphogenetic protein 4 + 5 ng / ml vascular endothelial growth factor) continue to culture until the sixth day.
[0058] (3) The cells differentiated to D6 were digested with 0.25% trypsin-EDTA, collected and centrifuged, added to OP9-DL1 feeder cells for co-cultivation, and cultured in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com