A high-safety non-inactivated virus preservation solution and its preparation method
A high-safety, preservation solution technology, applied in the medical field, can solve problems such as virus infection, achieve the effects of reducing infectivity, improving safety, and strengthening inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
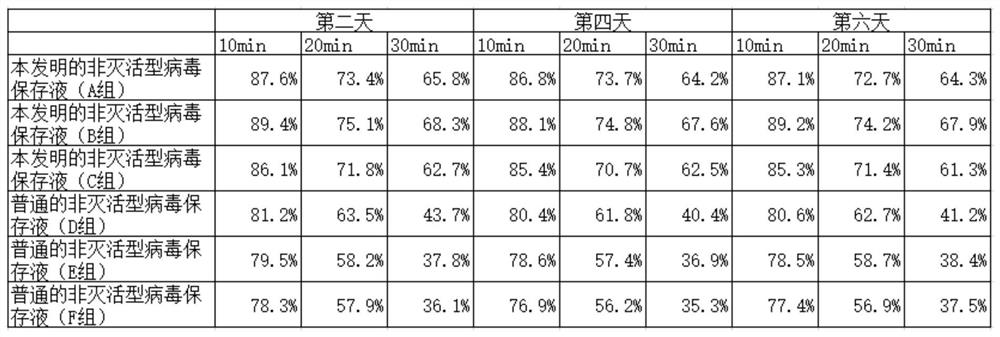
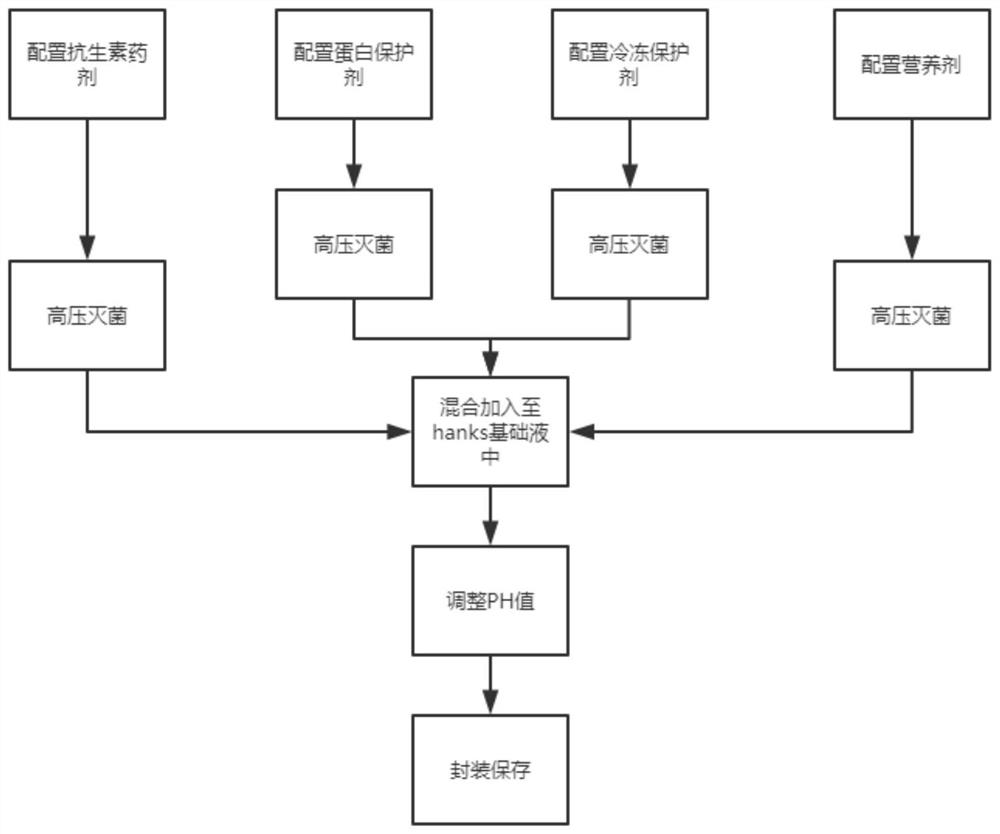
[0030] A high-safety non-inactivated virus preservation solution is characterized in that it includes hanks base solution, antibiotic medicament, protein protectant, cryoprotectant and nutritional agent, and the protein protectant includes bovine serum albumin and mannitol , glycerin, butylene glycol, sodium chloride and sodium benzoate, the cryoprotectant includes sodium alginate, polyethylene glycol, glucose, and the nutrient includes asparagine, glutamine, threonine, aspartame amino acid, valine, leucine, isoleucine, said antibiotic agents include gentamicin, amclocillin sodium and amikacin;
[0031] The weight ratio of each substance in the non-inactivated virus preservation solution is: 100 parts of Hanks base solution, 4 parts of antibiotic medicament, 3 parts of protein protection agent, 1 part of cryoprotectant and 1 part of nutrient;
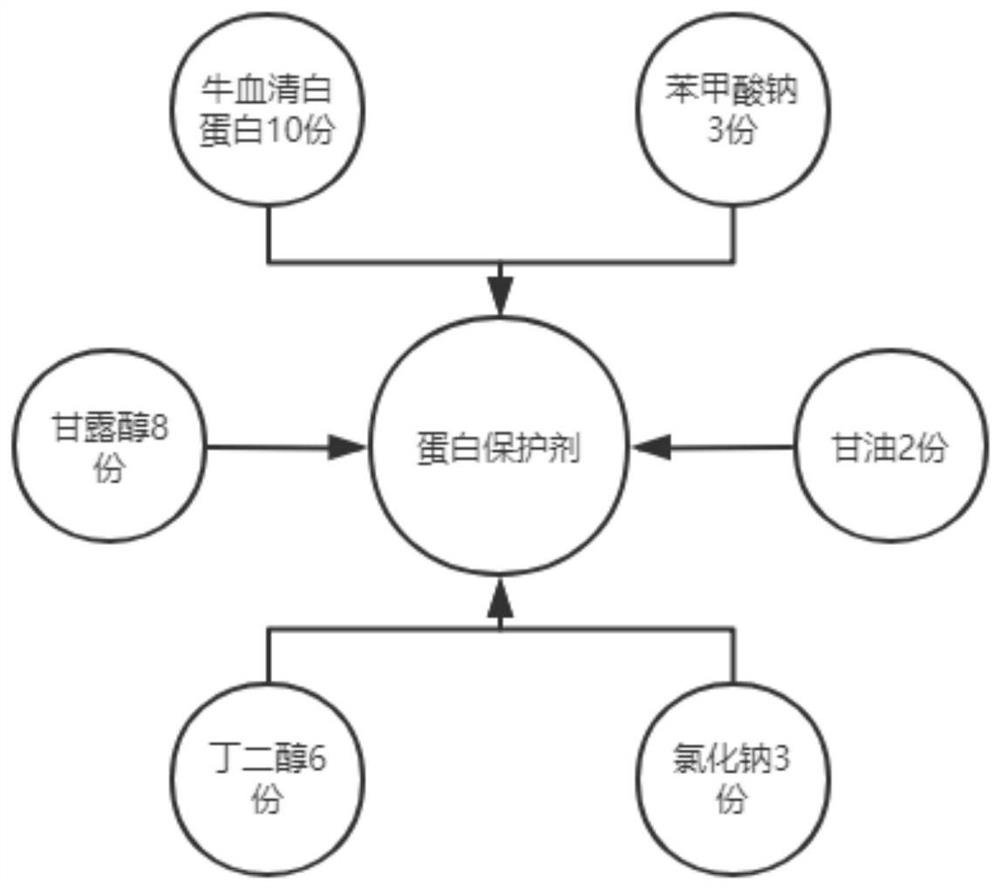
[0032] The weight ratio of each substance in the protein protectant is: 10 parts of bovine serum albumin, 8 parts of mannitol, 2 parts...
Embodiment 2
[0037] A high-safety non-inactivated virus preservation solution is characterized in that it includes hanks base solution, antibiotic medicament, protein protectant, cryoprotectant and nutritional agent, and the protein protectant includes bovine serum albumin, mannitol , glycerin, butylene glycol, sodium chloride and sodium benzoate, the cryoprotectant includes sodium alginate, polyethylene glycol, glucose, and the nutrient includes asparagine, glutamine, threonine, aspartame amino acid, valine, leucine, isoleucine, said antibiotic agents include gentamicin, amclocillin sodium and amikacin;
[0038] The weight ratio of each substance in the non-inactivated virus preservation solution is: 100 parts of Hanks base solution, 4 parts of antibiotic medicament, 3 parts of protein protection agent, 1 part of cryoprotectant and 1 part of nutrient;
[0039] The weight ratio of each substance in the protein protectant is: 10 parts of bovine serum albumin, 8 parts of mannitol, 2 parts of...
Embodiment 3
[0051] A high-safety non-inactivated virus preservation solution is characterized in that it includes hanks base solution, antibiotic medicament, protein protectant, cryoprotectant and nutritional agent, and the protein protectant includes bovine serum albumin, mannitol , glycerin, butylene glycol, sodium chloride and sodium benzoate, the cryoprotectant includes sodium alginate, polyethylene glycol, glucose, and the nutrient includes asparagine, glutamine, threonine, aspartame amino acid, valine, leucine, isoleucine, said antibiotic agents include gentamicin, amclocillin sodium and amikacin;
[0052] The weight ratio of each substance in the non-inactivated virus preservation solution is: 100 parts of Hanks base solution, 4 parts of antibiotic medicament, 3 parts of protein protection agent, 1 part of cryoprotectant and 1 part of nutrient;
[0053] The weight ratio of each substance in the protein protectant is: 10 parts of bovine serum albumin, 8 parts of mannitol, 2 parts of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com