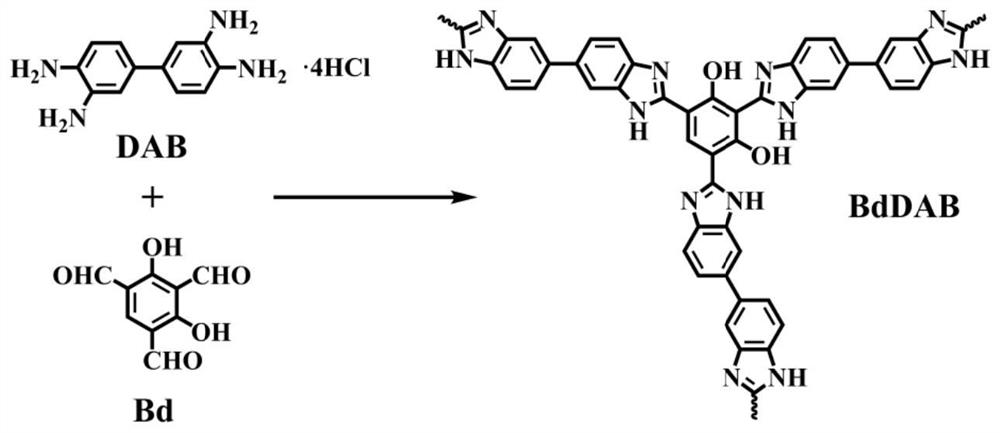
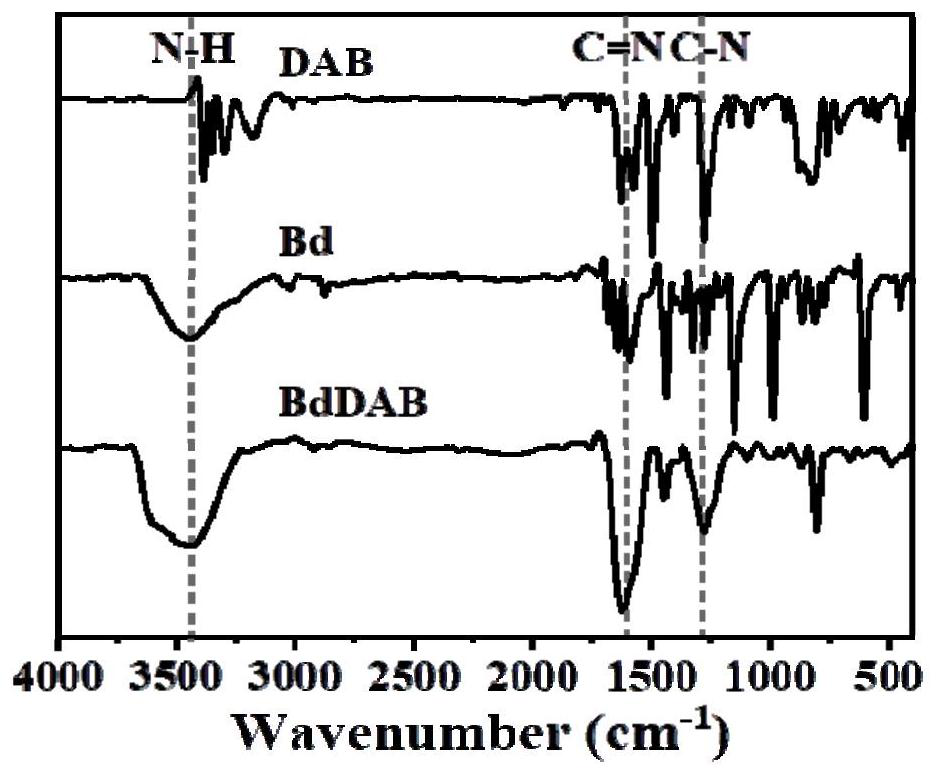
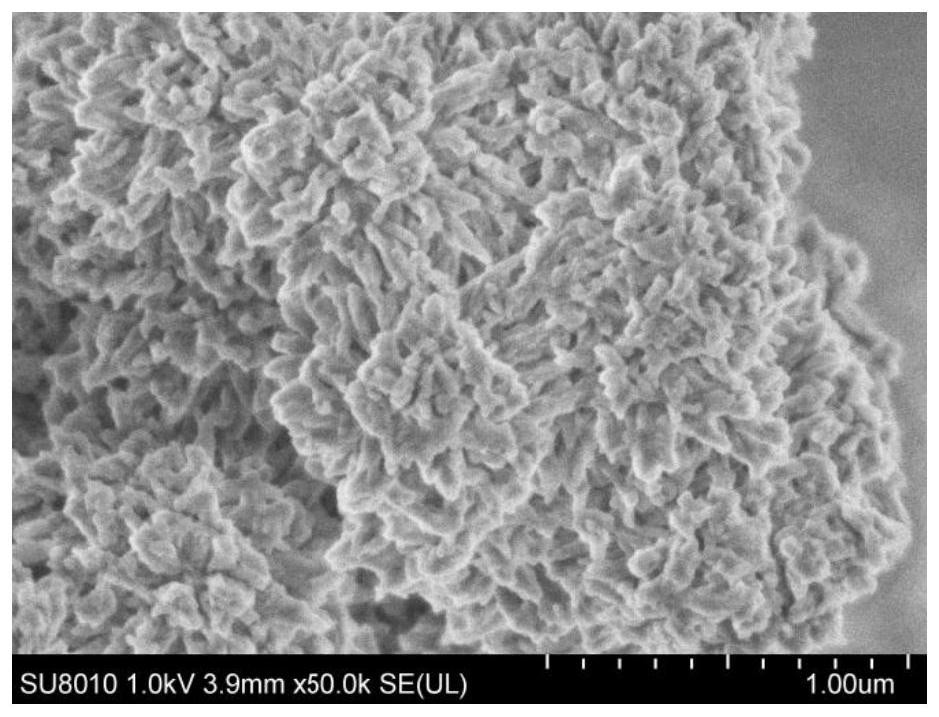
Preparation method of heterocyclic covalent organic polymer and application of heterocyclic covalent organic polymer in uranyl ion adsorption
A covalent organic and uranyl ion technology, applied in ion exchange, selective adsorption, ion exchange regeneration, etc., has achieved good application prospects, simple preparation method, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation method and property characterization of heterocyclic covalent organic polymer (BdDAB)
[0030]2,4,6-trialdehyde resorcinol (Bd, 0.048g, 0.25mmol), 3,3'-diaminodiphenylamine (DAB, 0.082g, 0.38mmol), 1,4-dioxane (4.0mL) and mesitylene (2.0mL) were put into a 20mL Pyrex glass tube, sonicated for 10 minutes, and then HAc solution (0.5mL, 6M) was added to obtain a reaction mixture solution; After the Pyrex glass tube of the solution was degassed through three freezing pump-thawing cycles, the Pyrex glass tube was flame-sealed and heated in an oven at 120°C for 3 days. After cooling, the brick-red product was separated by filtration and washed with After washing several times with anhydrous tetrahydrofuran (THF), N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMA), the solid was collected and dried under vacuum at 60°C for 8 hours to obtain imidazole 2,4,6-trialdehyde resorcinol-3,3'-diaminodianiline heterocyclic covalent organic polymer (BdDAB) w...
Embodiment 2
[0035] Embodiment 2: optimization of experimental conditions
[0036] The pH value has an effect on the basic form of uranium element and the extraction capacity of the adsorbent, so the effect of pH (1.0-4.5) on the performance of the adsorbent was studied. Use nitric acid or sodium hydroxide solution to adjust the pH value of the solution within the range of 1.0-4.5, add 4 mg of heterocyclic covalent organic polymer to 20 mL of aqueous solution with a concentration of uranyl ion of 75 mg / L, and use a constant temperature oscillator to shake for 12 After 1 hour, filter with a 0.22 μm microporous membrane, measure the remaining uranyl ion content in the filtrate by inductively coupled plasma mass spectrometry, and calculate the adsorption capacity of heterocyclic covalent organic polymers for uranyl ions. Figure 4 is the effect of pH on adsorption of UO by heterocyclic covalent organic polymer BdDAB 2 2+ Performance Impact Diagram. From Figure 4 It can be seen that the a...
Embodiment 3
[0037] Example 3: Heterocyclic Covalent Organic Polymer BddDAB to UO 2 2+ adsorption and removal
[0038] The effect of the initial concentration of uranium and the adsorption time on the adsorption of UO by the heterocyclic covalent organic polymer BdDAB was studied 2 2+ Impact. Use nitric acid or sodium hydroxide solution to adjust the pH of the solution to 4.5, add 4mg of heterocyclic covalent organic polymer BdDAB to 20mL containing different concentrations of UO 2 2+ (25-280mg / L) aqueous solution, use a constant temperature oscillator to shake for 12 hours, filter with a 0.22μm microporous membrane, and use inductively coupled plasma mass spectrometry to measure the remaining UO in the filtrate 2 2+ content, calculated for heterocyclic covalent organic polymers to UO 2 2+ The adsorption capacity of the heterocyclic covalent organic polymer BdDAB versus UO 2 2+ The adsorption isotherm. Figure 5 is the heterocyclic covalent organic polymer BdDAB on UO 2 2+ The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com