Kit for detecting anti-mutant citrullinated waveform protein antibody and detection method
A citrullination and vimentin technology, applied in the field of immunoassay, can solve the problems of complicated operation process, long experiment time, low accuracy and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
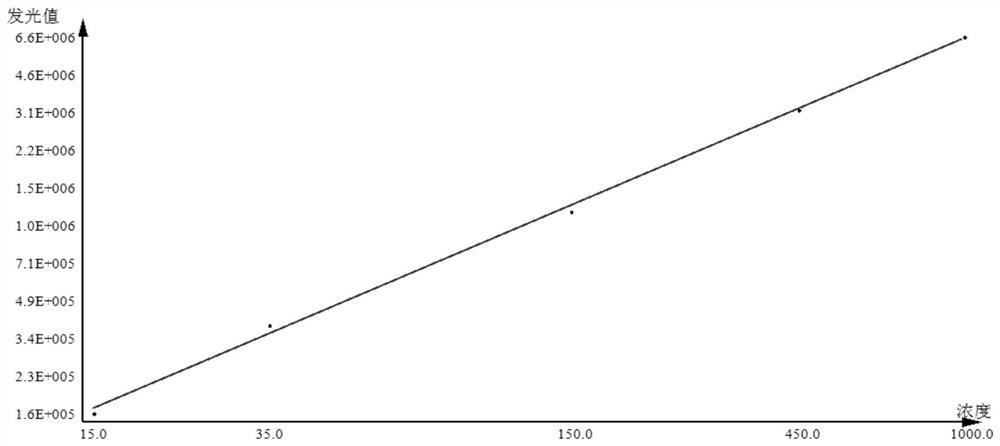
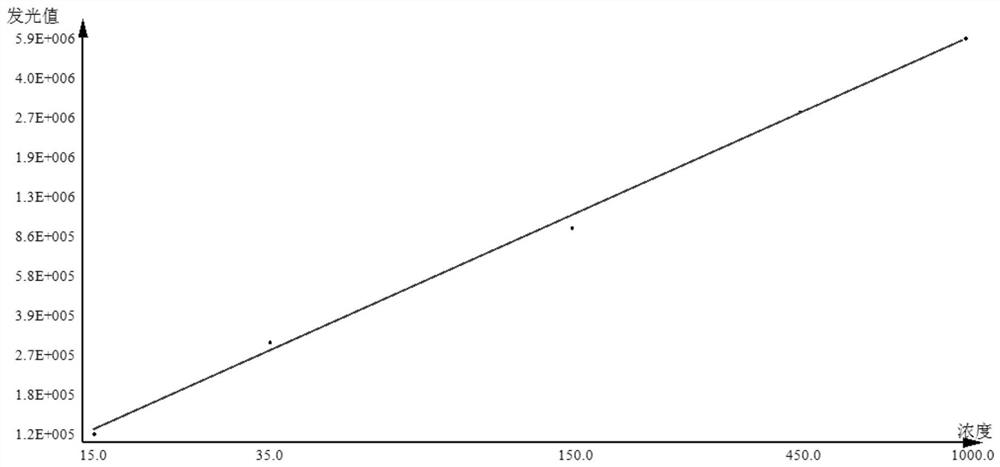
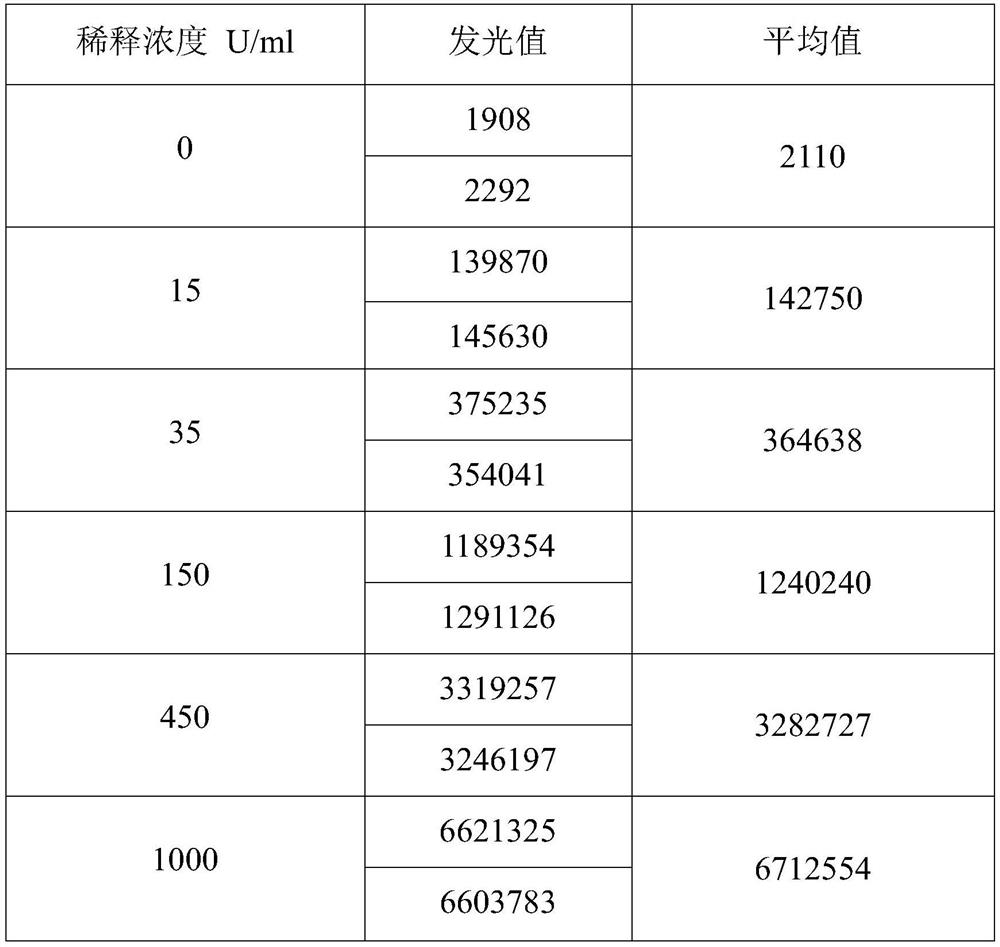
[0039] This embodiment provides a kit for detecting anti-MCV antibodies, which includes: streptavidin-labeled magnetic particle solution, biotin-labeled mutant citrullinated vimentin solution, acridinium ester-labeled mouse anti-human antibody solutions, substrates compatible with acridinium esters, calibrators, controls, and sample diluents.
[0040] Wherein, the substrate includes excitation solution A and excitation solution B, and excitation solution A contains: 0.07mol / L HCl and 0.65wt% H 2 o 2 , pH 1.2; excitation solution B contains: 0.6mmol / L NaOH and 0.65wt% dodecyltrimethylammonium iodide, pH 13.2.
[0041] The above-mentioned streptavidin-labeled magnetic particle solution was prepared by the following method:
[0042] (1) Take amino-modified magnetic particles (particle size: 0.1-5 μm) for terminal modification and activation: take 50 mg of amino-modified magnetic particles in a beaker, add an equal volume of acetone and methanol 2 ml, wash 3 times, magnetically ...
Embodiment 2
[0057] This embodiment provides a method for detecting anti-MCV antibodies using the kit of Embodiment 1, which includes the following steps:
[0058] (1) When the test sample is collected serum or plasma (handle the sample to avoid contamination, the frozen sample must be completely thawed, and then fully mixed), draw 10 μl of the sample and dilute it 10 times with the sample diluent, and mix it for later use;
[0059] (2) Take 50 μl of the diluted sample, add 50 μl of streptavidin-labeled magnetic particle solution, 50 μl of biotin-labeled mutant citrullinated vimentin solution, and 50 μl of acridinium ester-labeled mouse anti-human antibody solution, Incubate at 37°C for 18 minutes.
[0060] (3) Wash 3 times with washing solution after incubation, the washing solution is sodium chloride containing 2mol / L, 2wt% Tween-20 and 0.1mol / L-1mol / L phosphate buffer, buffer The pH is 7.8.
[0061] (4) After washing, add 100 μl of pre-excitation solution A, and then add 100 μl of exc...
Embodiment 3
[0063] The composition of the kit for detecting anti-MCV antibodies provided in this example is basically the same as that in Example 1, the difference is that this example uses alkaline phosphatase-labeled mouse anti-human antibody as the labeled antibody, corresponding to the chemiluminescence substrate used Be (adamantane)-1,2-dioxetane (AMPPD); 7.00-9.50), 0.2mol / L sodium sulfate, 0.1mol / L sodium chloride, 0.2wt% Proclin-300 and 2wt% luminescence enhancer.
[0064] The preparation method of the above-mentioned alkaline phosphatase-labeled mouse anti-human antibody is as follows:
[0065] Weigh 25 mg of alkaline phosphatase in glutaraldehyde solution, mix thoroughly, and place it at room temperature overnight. The reacted solution is eluted with sodium carbonate solution (0.2mol / L, pH 9.0) through a chromatographic column. Collect the effluent liquid, dissolve 12.5mg of mouse anti-human antibody in sodium carbonate solution (0.2mol / L, pH 9.0), stir until completely dissolv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com