Method for degrading phenol by nitrogen-boron co-doped carbon-based microbial fuel cell cathode
A fuel cell cathode and co-doping technology, which is applied in biochemical fuel cells, chemical instruments and methods, biological water/sewage treatment, etc., can solve the problems of ecological environment and human health threats, phenol residue, etc., and achieve improved electrocatalytic performance , excellent stability, easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0021] Specific embodiment one: a kind of nitrogen-boron co-doped carbon-based microbial fuel cell cathode degradation method for phenol in this embodiment is carried out according to the following steps;
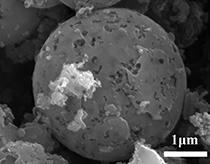
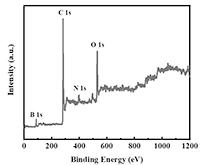
[0022] 1. Preparation of BNBC cathode catalyst: 0.299g of CoCl 2 ·6H 2 O, 0.1166g of boric acid and 0.6g of melamine (molar ratio 1:1.5:3) were dissolved in 50mL of deionized water, then 2g of dried pomelo peel powder was added to it, and stirred until completely mixed; the above solution was added Hydrothermally heat at 180° C. for 12 hours in a polytetrafluoroethylene autoclave, and put the hydroheated product in an oven at 60° C. to dry to remove moisture. Subsequently, the obtained solid was transferred to a tube furnace and heated under N 2 Atmosphere, at 2°C min –1 The heating rate was heated from room temperature to 900 °C, and kept at the target temperature for 2 h to obtain a carbonized black product. Immerse the black sample with 0.5M nitric acid and put it in...
specific Embodiment approach 2
[0028] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the CoCl described in step one 2·6H 2 O: The molar ratio of boric acid: melamine is 1:3:3, and the other is the same as the first embodiment.
specific Embodiment approach 3
[0029] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: the CoCl described in step one 2 ·6H 2 O: The molar ratio of boric acid: melamine is 1:6:3, and the others are the same as the specific embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com