Rutin-pyruvaldehyde adduct, preparation method and application
A technology of pyruvic aldehyde adduct and rutin, which can be used in measuring devices, instruments, scientific instruments, etc., can solve problems such as food safety risks, and achieve the effects of safety evaluation, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
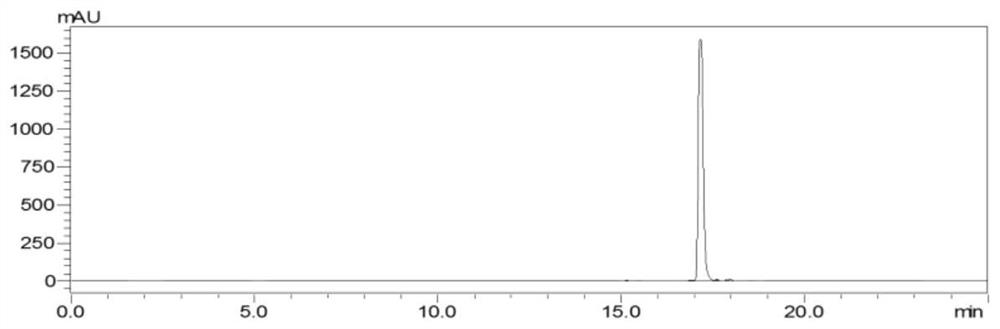
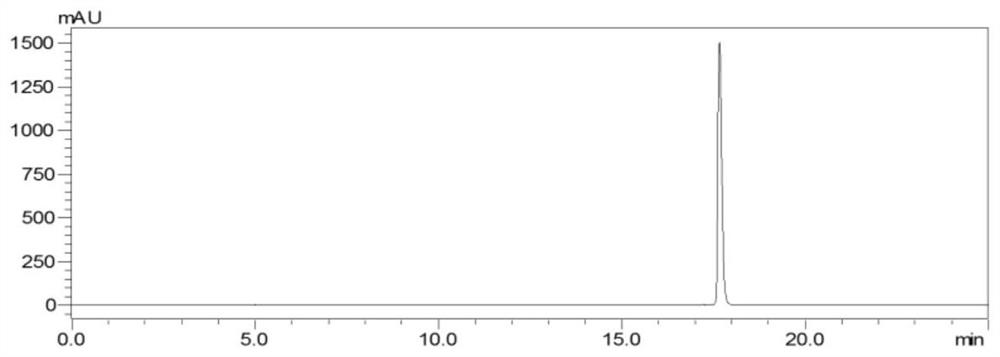
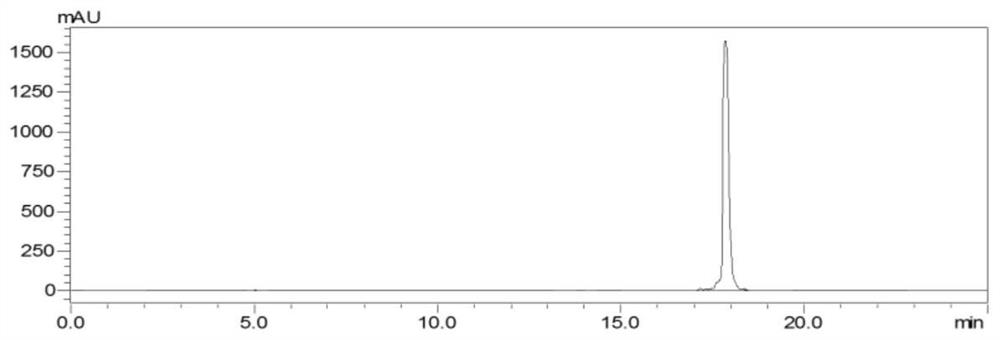
[0076] The preparation method of three kinds of rutin-methylglyoxal adducts comprises the following steps: in 0.2M phosphate buffered saline, rutin and methylglyoxal are reacted in the dark according to the molar ratio of 1:10 (reaction at 37°C for 24h, rotation speed 20rpm), concentrated under reduced pressure to 1-2mL, passed through a 0.45μm filter membrane to obtain the filtrate, desalted by gel column chromatography and purified by reverse-phase silica gel chromatography, and the fractions collected after purification were analyzed by HPLC. Pure, the same substances were combined, spin-dried to elute the solvent, and the powder product was collected after freeze-drying at -70°C and 10Pa for 48 hours to obtain three rutin-methylglyoxal adducts: compound a, compound b and compound c, three kinds of rutin The yields of the glycoside-acetoglyoxal adducts were a: 28.5%, b: 29.4%, and c: 10.4%, respectively.
[0077] The method for desalination by gel column chromatography desc...
Embodiment 2
[0100] The preparation method of the rutin-methylglyoxal adduct comprises the following steps: in 0.2M phosphate buffered saline, rutin and methylglyoxal are subjected to a dark-light heating reaction according to a molar ratio of 1:5 (reaction at 70°C for 12h, rotating speed 20rpm ), concentrated under reduced pressure to 1-2 mL, passed through a 0.45 μm filter membrane to obtain the filtrate, purified by reverse-phase silica gel chromatography after desalination by gel column chromatography, and purified by HPLC for the fractions collected after purification. The same substances were combined, spin-dried to elute the solvent, and the powder product (that is, rutin-acetoglyoxal adduct) was collected after freeze-drying at -50°C and 20 Pa for 48 hours. The purity of compound, compound b and compound c were 98.0%, 98.0%, and 97.5%, 98.6%, the yields of the three rutin-methylglyoxal adducts are respectively a: 15.4%, b: 14.3%, c: 33.0%.
[0101] The steps of desalting by gel col...
Embodiment 3
[0104] The preparation method of rutin-methylglyoxal adduct comprises the following steps: in 0.2M phosphate buffered saline, rutin and methylglyoxal are subjected to dark heating reaction according to the molar ratio of 1:10 (reaction at 70°C for 13h, rotating speed 20rpm ), concentrated under reduced pressure to 1-2 mL, passed through a 0.45 μm filter membrane to obtain the filtrate, purified by reverse-phase silica gel chromatography after desalination by gel column chromatography, and purified by HPLC for the fractions collected after purification. The same substances were combined, spin-dried to elute the solvent, and the powder product (that is, rutin-acetoglyoxal adduct) was collected after freeze-drying at -40°C and 10 Pa for 48 hours. The purity of compound, compound b and compound c were 97.2%, 97.2%, and 98.0%, 98.3%, the yields of the three rutin-methylglyoxal adducts are respectively a: 17.6%, b: 22.8%, c: 43.6%.
[0105] The steps of desalting by gel column chrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com