Method for preparing eribulin intermediate through micro-channel reactor
A technology of microchannel reactors and intermediates, applied in chemical instruments and methods, chemical/physical/physical chemical reactors, chemical/physical processes, etc., can solve problems such as high energy consumption, cumbersome operations, and low reaction yields , to achieve the effect of increasing the reaction temperature, shortening the reaction time, and reducing the energy consumption of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
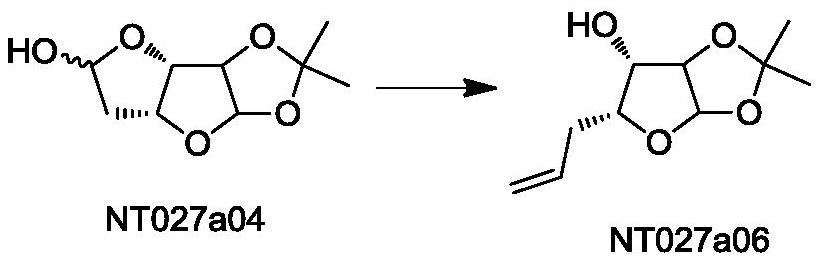
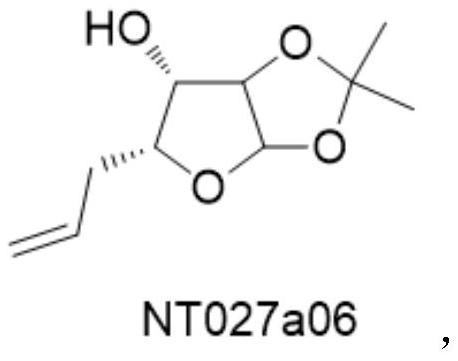
[0030] The preparation of embodiment 1 compound NT027a06
[0031] Weigh 35 grams of NT027a04 (0.173 moles) and 185.9 grams of triphenylmethylphosphine bromide (0.519 moles), add them into 1000 mL of tetrahydrofuran and stir, and dissolve fully to form material I, which is transported to reaction module 1;
[0032] In addition, 138.4 ml of 2.5M n-butyl lithium in n-hexane (0.346 mol) was taken as material II and transported to reaction module 2;
[0033] Adjust the slurry pump so that the flow rate of material I is 10.6 ml / min, and the flow rate of material II is 2.2 ml / min. The two materials are mixed and reacted at 30°C in the mixing module, and the residence time is 80 seconds. The reaction liquid at the outlet is collected , quenched the reaction by adding saturated brine, and extracted the aqueous phase with ethyl acetate after separation. The organic phases were combined, spin-dried and purified by column chromatography to obtain 31.6 g of NT027a06, with a yield of 91.2%....
Embodiment 2
[0034] The preparation of embodiment 2 compound NT027a06
[0035] Weigh 33 grams of NT027a04 (0.163 moles) and 192.4 grams of triphenylmethylphosphine bromide (0.539 moles), add them to 1000 mL of tetrahydrofuran and stir, and dissolve fully to form material I, which is transported to reaction module 1;
[0036] In addition, 196 milliliters of 2.5M n-butyl lithium in n-hexane (0.490 moles) was taken as material II and transported to reaction module 2;
[0037] Adjust the slurry pump so that the flow rate of material I is 10.7 ml / min, and the flow rate of material II is 2.1 ml / min. The two materials are mixed and reacted at 50°C in the mixing module, and the residence time is 30 seconds. The reaction liquid at the outlet is collected , quenched the reaction by adding saturated brine, and extracted the aqueous phase with ethyl acetate after separation. The organic phases were combined, spin-dried and purified by column chromatography to obtain 31.1 g of NT027a06 with a yield of ...
Embodiment 3
[0038] The preparation of embodiment 3 compound NT027a06
[0039] Weigh 30 grams of NT027a04 (0.148 moles) and 212.1 grams of triphenylmethylphosphonium bromide (0.592 moles), add them to 1000 mL of tetrahydrofuran and stir, and dissolve fully to form material I, which is transported to reaction module 1;
[0040] In addition, 236.8 ml of 2.5M n-butyl lithium in n-hexane solution (0.592 mol) was taken as material II and transported to reaction module 2;
[0041] Adjust the slurry pump so that the flow rate of material I is 11.8 ml / min, and the flow rate of material II is 2.1 ml / min. The two materials are mixed and reacted at 70°C in the mixing module, and the residence time is 20 seconds. The reaction liquid at the outlet is collected , quenched the reaction by adding saturated brine, and extracted the aqueous phase with ethyl acetate after separation. The organic phases were combined, spin-dried and purified by column chromatography to obtain 26.6 g of NT027a06, with a yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com