Application of sulfonated polyaryletherketone as binder in membrane electrode of proton exchange membrane fuel cell, membrane electrode and preparation method
A sulfonated polyaryl ether ketone and proton exchange membrane technology, applied in fuel cells, battery electrodes, circuits, etc., can solve problems that are not conducive to the electrochemical performance of proton exchange membrane fuel cells, reduce the electrochemical active area of electrodes, and reduce solvent residue more questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] The present invention also provides a method for preparing the membrane electrode described in the above technical solution, comprising the following steps:
[0083] Mixing the sulfonated polyaryletherketone solution and the catalyst to obtain a catalyst slurry, the solvent in the sulfonated polyaryletherketone solution is a low boiling point solvent, and the boiling point of the low boiling point solvent is lower than 100°C;
[0084] Coating the catalyst slurry on both sides of the proton exchange membrane, removing the solvent in the sulfonated polyaryletherketone solution, forming a first catalyst layer and a second catalyst layer on both sides of the proton exchange membrane to obtain a primary membrane electrode ;
[0085] Composite the first gas diffusion layer and the second gas diffusion layer on both sides of the primary membrane electrode to obtain the membrane electrode.
[0086] The invention mixes the sulfonated polyaryletherketone solution and the catalys...
Embodiment 1
[0096] 4.3641g of 4,4'-difluorobenzophenone, 12.6687g of 4,4'-difluorobenzophenone-3,3'-disodium sulfonate, 12.1152g of 3,3', Mix 5,5'-tetramethyl-4,4'-dihydroxybiphenyl, 87.44g sulfolane and 7.59g anhydrous potassium carbonate, heat up to 140°C for 3 hours under nitrogen protection, and heat up at a rate of 0.3°C / mn React at 210°C for 3 hours to obtain sulfonated polyaryletherketone sodium salt;
[0097] The obtained sulfonated polyaryletherketone sodium salt and 1mol / L of H 2 SO 4 Mix to carry out displacement reaction on the sodium salt of sulfonated polyaryletherketone. After the displacement reaction is completed, the solid product is ground, washed repeatedly with water, and then dried at 60° C. for 24 hours to obtain the sulfonated polyaryletherketone.
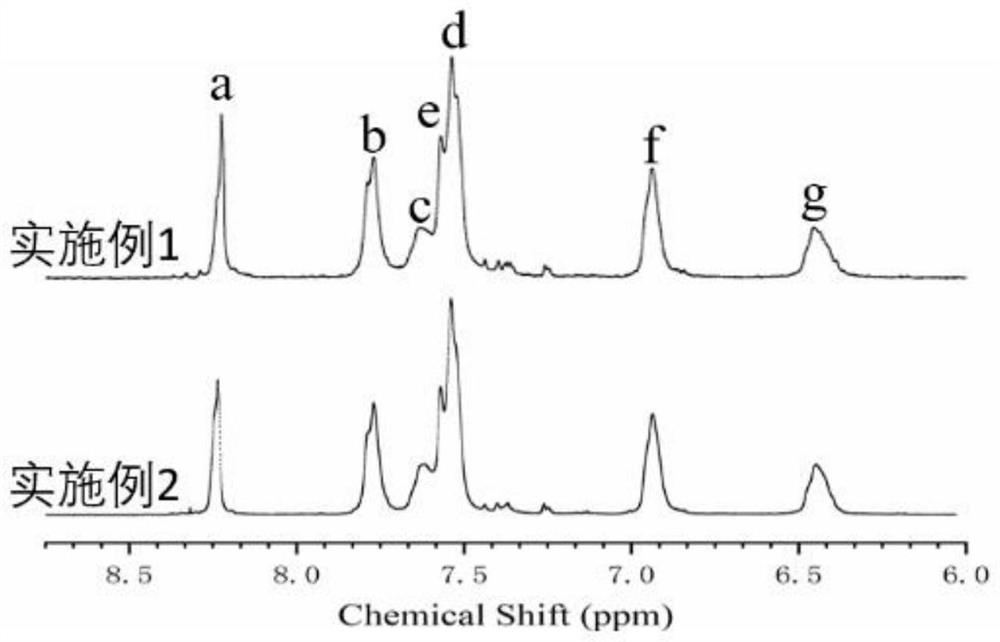
[0098] The obtained sulfonated polyaryletherketone of embodiment 1 is carried out nuclear magnetic resonance test, and gained nuclear magnetic resonance spectrum figure is shown in figure 1 . Depend on figure 1 It ...
Embodiment 2
[0106] 10.9103g of 4,4'-difluorobenzophenone, 12.1152g of 3,3',5,5'-tetramethyl-4,4'-dihydroxybiphenyl, 69.07g of sulfolane and 7.59g of Mix with water and potassium carbonate, raise the temperature to 140°C for 3 hours under the protection of nitrogen, then raise the temperature to 210°C for 3 hours at a rate of 0.6°C / min, pour the obtained product system into water, grind the obtained solid product, and wash with water repeatedly After drying at 80°C for 12 hours, polyaryletherketone was obtained;
[0107] Dissolve 10g of polyaryletherketone in 150mL of concentrated sulfuric acid, react at 80°C for 6h, pour the product system into ice water, filter, adjust the solid product to pH 8 with NaOH, wash with deionized water until neutral, and Dry at 60°C for 24 hours to obtain sulfonated polyaryletherketone sodium salt with a sulfonation degree of 1.2;
[0108] Soak the obtained sulfonated polyarylether ketone sodium salt in 60°C, 1mol / L H 2 SO 4 The replacement reaction was ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionic conductivity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com