Combination vaccine composition comprising reduced dose inactivated poliovirus and method for preparing the same
A composition and inactivation technology, applied in biochemical equipment and methods, medical preparations without active ingredients, medical preparations containing active ingredients, etc., can solve the problems of saturated adsorption capacity and incomplete adsorption of antigens.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
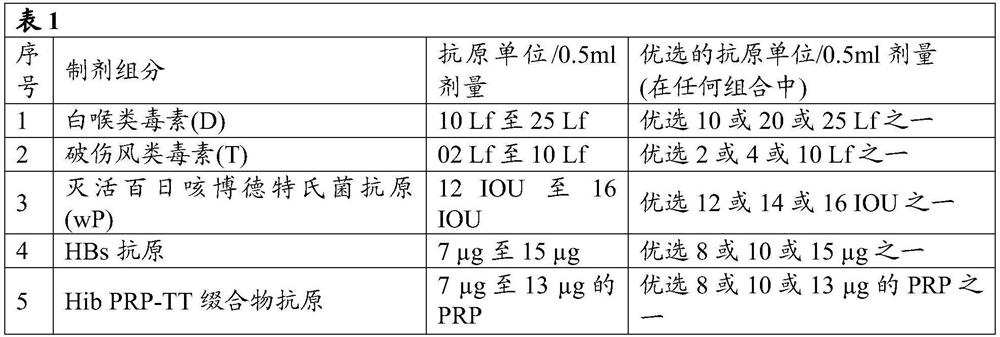
[0090] According to a first embodiment of the present disclosure, the combination vaccine composition includes, but is not limited to, an antigen / immunogen selected from the group consisting of Diphtheria Toxoid (D), Tetanus Toxoid (T), Whole Cell Pertussis Bordet influenzae (wP), Haemophilus influenzae type b (Hib) PRP-CP conjugate, hepatitis B (HepB), reduced doses of inactivated poliovirus (IPV), and additionally 2-phenoxyethanol and at least one A combination of paraben preservatives.
[0091] According to the second embodiment of the present disclosure, the combination vaccine composition may further include one or more antigens, which are respectively selected from but not limited to the group consisting of: Haemophilus influenzae (a, c, d , e, f serotypes and acapsulated strains), hepatitis (A, C, D, E, F and G strains), Neisseria meningitidis A, B, C, Y, W-135 or X, Influenza, Staphylococcus aureus, Salmonella typhi antigen, acellular pertussis antigen, modified adeny...
no. 5 approach
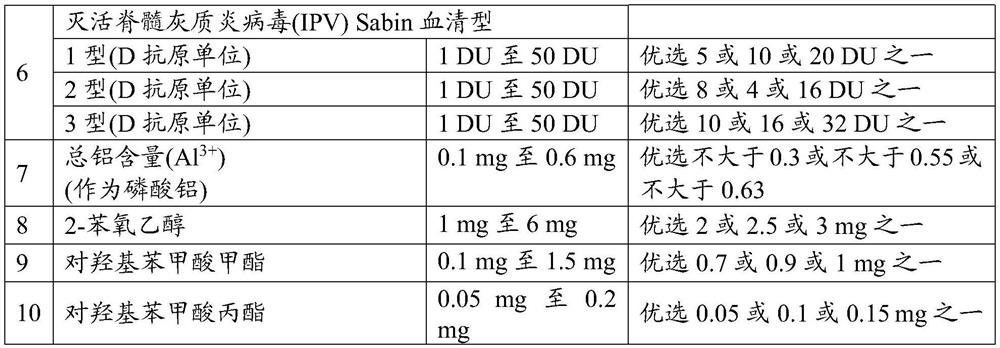
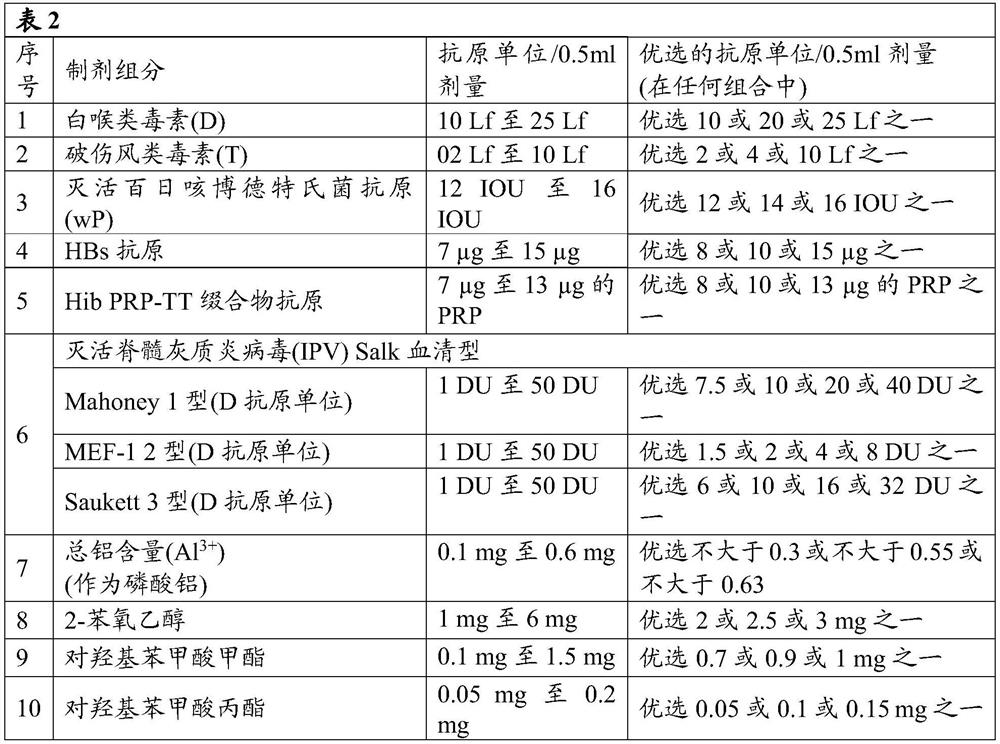
[0108] According to the fifth embodiment of the present disclosure, IPV (Sabin strain or Salk strain) may not be adsorbed on any adjuvant alone and then added to the final combination vaccine composition.
[0109] According to a preferred aspect of the fifth embodiment, IPV (Sabin strain or Salk strain) can be adsorbed on an adjuvant, more preferably on aluminum phosphate or aluminum hydroxide present in the combination vaccine, wherein the IPV antigen, for The percent adsorption of IPV type 1 can range from 10% to 30%, the percent adsorption of IPV type 2 can range from 60% to 100%, and the percent adsorption of IPV type 3 can range from 0% to 25% Inside.
no. 6 approach
[0110] According to the sixth embodiment of the present disclosure, IPV (Sabin strain or Salk strain) components can be adsorbed individually to an adjuvant selected from the group consisting of aluminum salt (Al 3+ ), such as aluminum hydroxide (Al(OH) 3 ) or aluminum phosphate (AlPO 4 ), alum, calcium phosphate, MPLA, 3D-MPL, QS21, CpG-containing oligodeoxynucleotide adjuvants, liposomes or oil-in-water emulsions or combinations thereof. (eg, before or after mixing with other ingredients, if present). If adsorbed, one or more IPV components can be adsorbed on aluminum hydroxide (Al(OH) 3 ) or aluminum phosphate.
[0111] IPV (Sabin strain or Salk strain) components can be adsorbed onto aluminum salts by the following procedure:
[0112] Take the desired volume of autoclaved Al(PO) 4 or Al(OH) 3 To achieve the final alum (Al 3+ ) at a concentration of 0.1 mg to 0.8 mg / dose,
[0113] • Add bulk IPV with adjusted D-Ag units and make up volume with diluent (10x M-199+0.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com