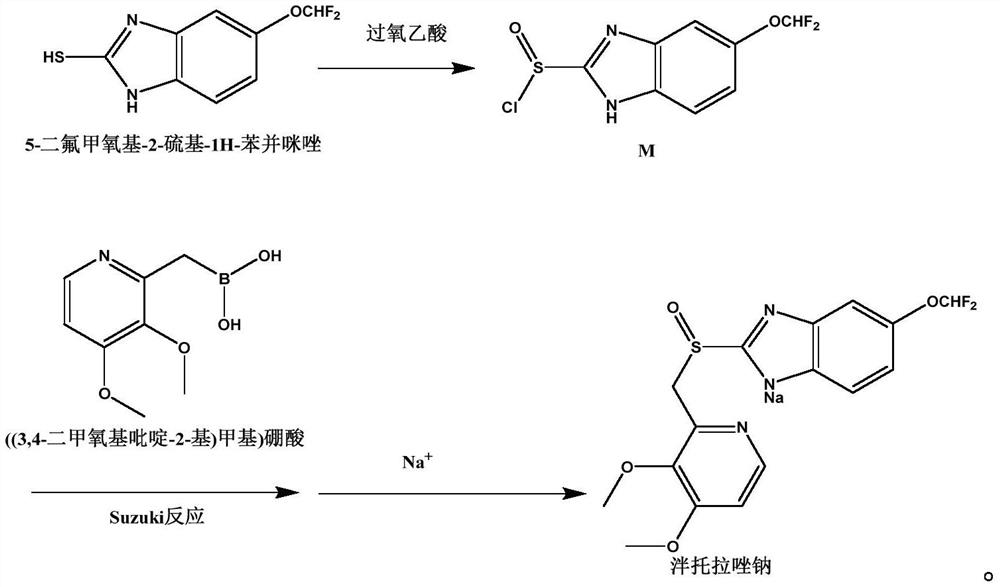
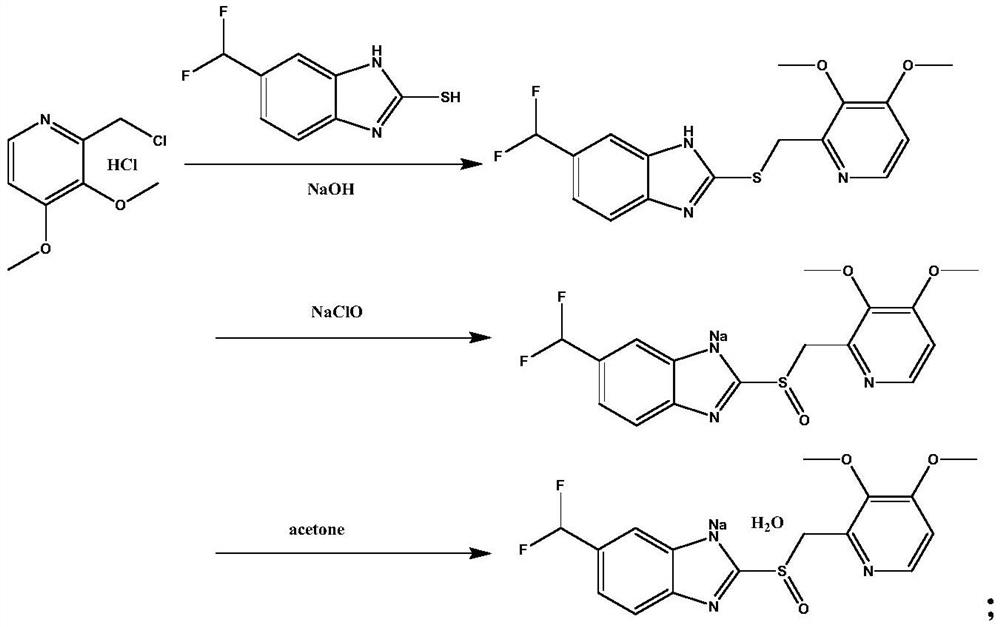
Synthetic method and application of pantoprazole sodium
A technology of pantoprazole sodium and a synthesis method, applied in the field of pharmaceutical synthesis, can solve the problems of difficult purification of intermediates and products, short technological process, low solubility, etc., and achieves improved raw material utilization, shortened technological process, simplified Effects of Synthesis Steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of synthetic method of embodiment 1 pantoprazole sodium A1
[0028] The present embodiment provides a kind of synthetic method of pantoprazole sodium A1, and its synthetic method comprises the following steps:
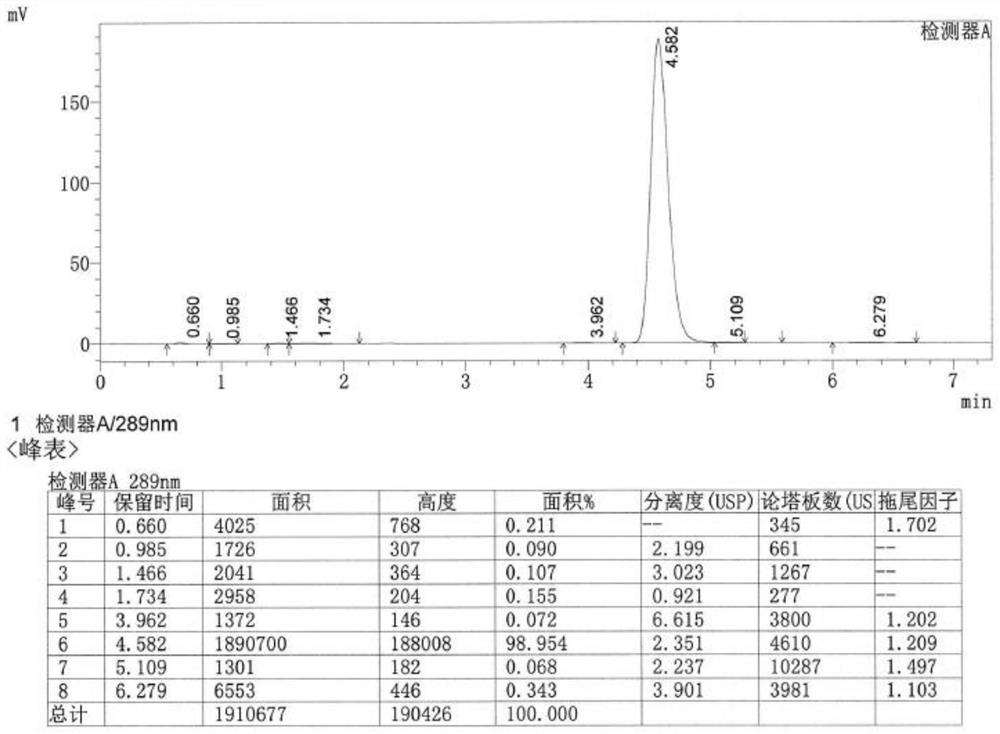
[0029] 1) Weigh 21.6g of 5-difluoromethoxy-2-sulfanyl-1H-benzimidazole into 100ml of dichloromethane, add 38g of 40% peracetic acid, and keep stirring at room temperature for 1h to carry out the sulfnylation reaction , TLC monitors the reaction, and the disappearance of the raw material point is the end of the reaction. The reaction solution is concentrated to an oily state at 30°C under reduced pressure to obtain 21.3g of intermediate M1 (yield is 81%), and its reaction formula is:
[0030]
[0031] 2) Weigh 21.3g of intermediate M1 and 18.9g of ((3,4-dimethoxypyridin-2-yl)methyl)boronic acid dissolved in 150ml of tetrahydrofuran, stir to dissolve, continue stirring and add 12.7g of sodium carbonate, Replace the air in the reaction vessel with nitrog...
Embodiment 2
[0033] A kind of synthetic method of pantoprazole sodium A2 of embodiment 2
[0034] The present embodiment provides a kind of synthetic method of pantoprazole sodium A2, and its synthetic method comprises the following steps:
[0035] 1) Weigh 21.6g of 5-difluoromethoxy-2-sulfanyl-1H-benzimidazole into 100ml of dichloromethane, add 38g of 40% peracetic acid, and keep stirring at room temperature for 1h to carry out the sulfonylation reaction. TLC monitors the reaction, and the disappearance of the raw material point is the end of the reaction. The reaction solution is concentrated under reduced pressure at 30°C to an oily state to obtain 21g of intermediate M2 (yield is 80%), and its reaction formula is:
[0036]
[0037] 2) Dissolve 21g of intermediate M2 and 18.9g ((3,4-dimethoxypyridin-2-yl)methyl)boronic acid in 150ml 1,4-dioxane, stir to dissolve, keep stirring and Add 21.6g of potassium carbonate, replace the air in the reaction vessel with nitrogen for three times,...
Embodiment 3
[0039] A kind of synthetic method of pantoprazole sodium A3 of embodiment 3
[0040] The present embodiment provides a kind of synthetic method of pantoprazole sodium A3, and its synthetic method comprises the following steps:
[0041] 1) Weigh 21.6g of 5-difluoromethoxy-2-sulfanyl-1H-benzimidazole into 100ml of dichloromethane, add 38g of 40% peracetic acid, and keep stirring at room temperature for 1h to carry out the sulfonylation reaction. TLC monitors the reaction, and the disappearance of the raw material point is the end of the reaction. The reaction solution is concentrated under reduced pressure at 30°C to an oily state to obtain 20.4g of intermediate M3 (yield is 79%), and its reaction formula is:
[0042]
[0043] 2) Weigh 20.4g of intermediate M3 and 22.7g of ((3,4-dimethoxypyridin-2-yl)methyl)boronic acid and dissolve in 150ml of 1,4-dioxane, stir to dissolve, keep stirring and Add 37.5g of cesium carbonate, replace the air in the reaction vessel with nitrogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com