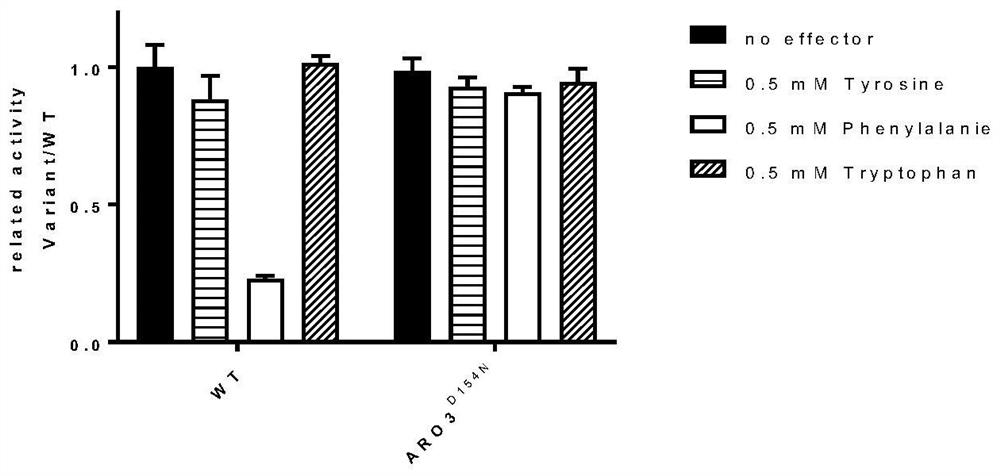
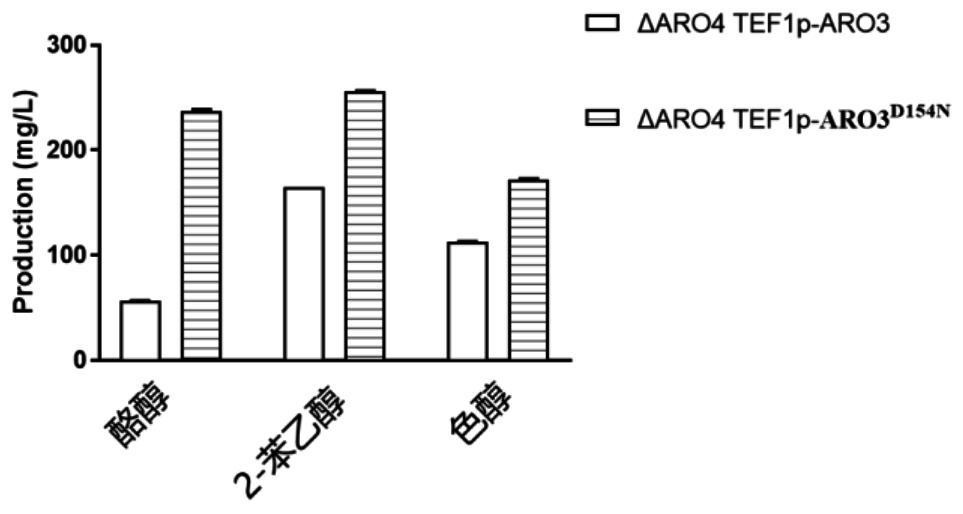
ARO3 protein mutant and application thereof
A mutant and protein technology, applied in ARO3 protein mutants and their application fields, can solve the problem of not finding mutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 ARO3 D154N Outer application of mutant protein and related plasmids, strains
[0041] Will encode ARO3 D154N The gene of the mutant (SEQ ID NO: 3) is inserted into the Escherichia coli expression vector PET21b to obtain a recombinant carrier.
[0042] The above recombinant vectors were converted to the expression of E. coli Rosetta (DE3).
[0043] The above strains were then tedped overnight, then transferred to Lb medium (100 μg / ml ampiconbin), and raised to OD. 600 = 0.6-0.8, the final concentration was 0.1 mmIPTG, 16 ° C, 110 rpm overnight induced expression. After collecting bacteria, centrifugation was collected after the breast, and then the mutant protein was collected using a phosphate buffer with low imidazole (20 mm imidazole) after incubation with nickel gel. The mutant protein was collected by a phosphate buffer with 250 mM imidazole. Phosphate buffer: 50 mM potassium hydrogen phosphate, pH is 6.5-6.8, 150 mM sodium chloride. The eluted protein dialy...
Embodiment 2
[0046] Example 2 ARO3 D154N Application of mutant protein and related plasmids, strains
[0047] 2.1 Sacchal yeast genome original position mutation ARO3 is ARO3 D154N
[0048] On the primary strain Cen.PK2-1 C strain genome, CRISPR / CAS9-mediated gene settling point mutation technique; the method of genetically fixed-point mutation, the plasmid of the plasmid carrying the Cas9 protein gene and a gene used to transcribe, transcribed RNA Combined with CAS9 protein, simply introduce a gene Spacer and homologous recombinant fragments that match the target gene on the plasmid, and will refer to the product into the cell, because the homologous recombinant fragment is fixed, and the yeast is eugenic The target gene in which the cell was cut, in homologous recombination, the homogeneous arm on the plasmid was a template, and the fixed-point mutation was completed, thereby completing the purpose of the genetically fixed point mutation.
[0049] Spacer: AcccattgctggTGAGATGT
[0050] Hom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com