Preparation method of stable lipoic acid tablet
A technology of lipoic acid tablets and lipoic acid, which is applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc., can solve problems such as increased production costs, increased polymer growth, and decreased stability and dissolution. , to achieve the effect of improving product stability, improving particle fluidity, and improving particle roundness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
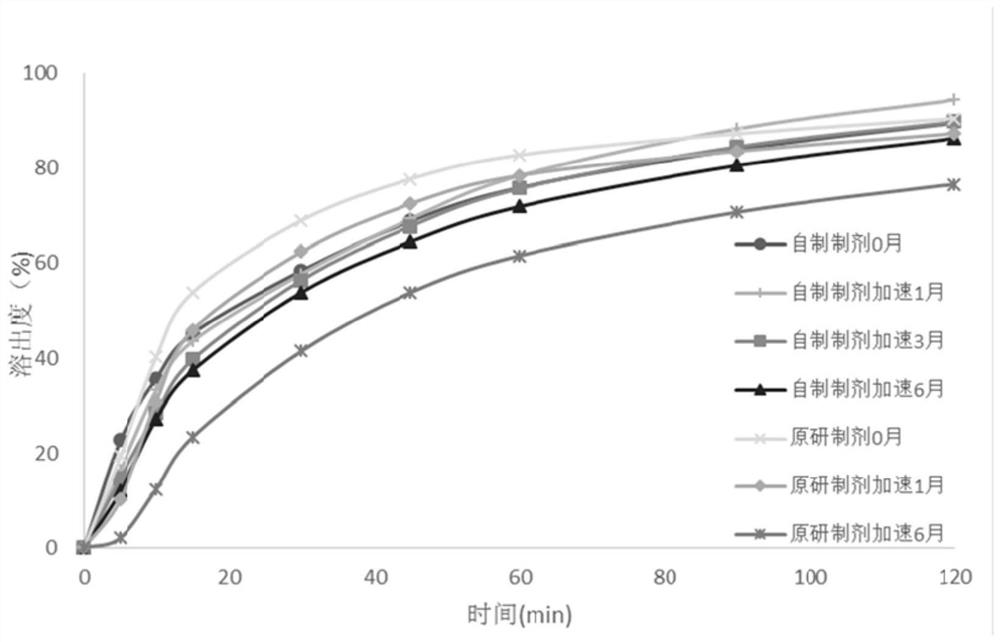
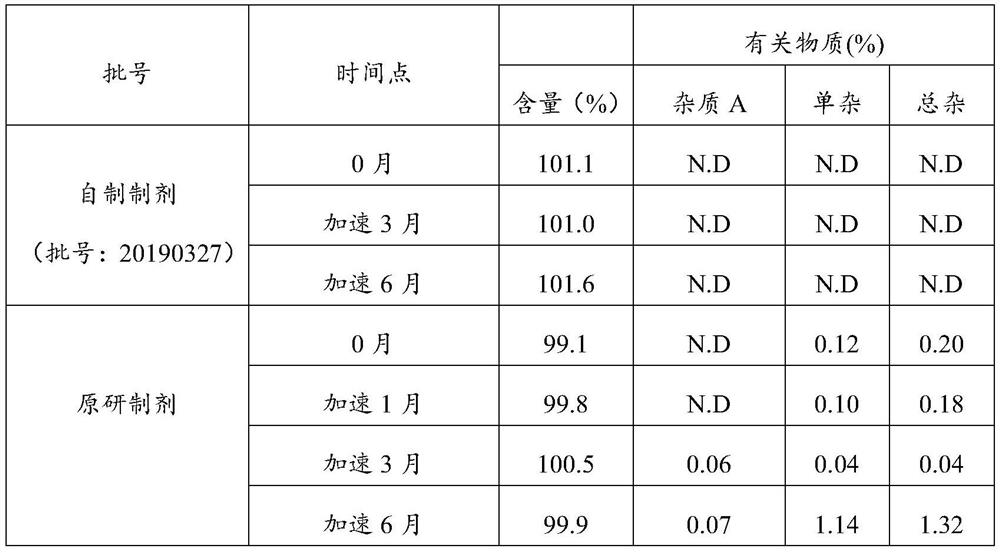
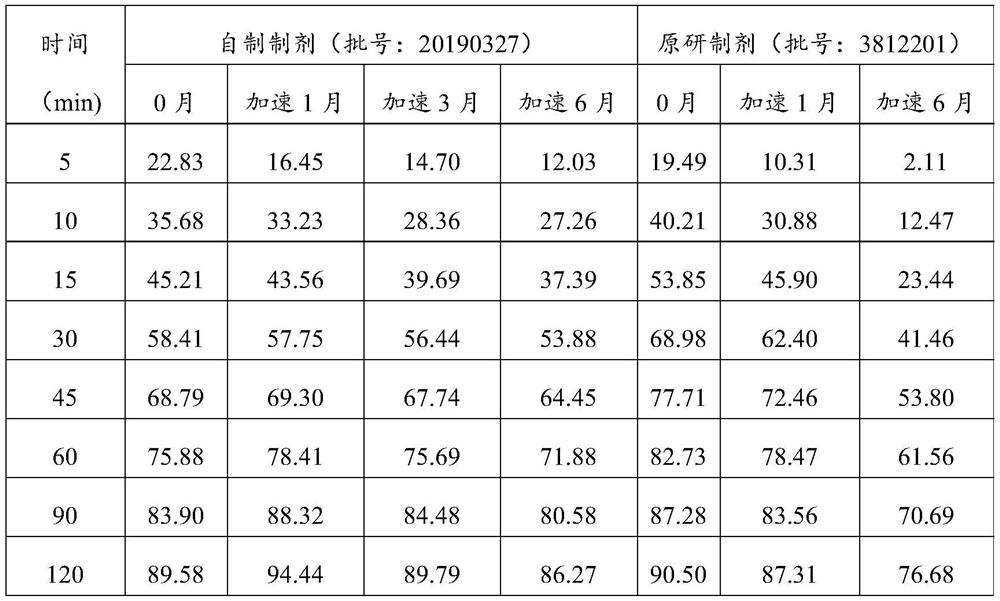
Image
Examples
Embodiment 1
[0032] Prepare 10% hydroxypropyl cellulose aqueous solution as adhesive. Weigh 600g of lipoic acid, place it in a wet granulator, turn on the stirring blade at 400rpm, and the shear knife at 2000rpm, mix for 1 minute, spray 200g of binder solution into granulation, pass through a 18-mesh sieve for granulation, and fluidized bed 40 ℃ drying for 30 minutes, 18-mesh sieve granulation.
[0033] Put the dry granules in a mixer, add 156g of low-substituted hydroxypropyl cellulose and 15g of magnesium stearate and mix for 5 minutes, then granulate with a dry granulator, set the pressure at 3MPa, the speed of the roller is 10rpm, and the speed of the granulator is 70rpm. 18 mesh sieve for granulation.
[0034] Add 9g of magnesium stearate, mix for 5 minutes, use special-shaped punched tablets with a diameter of 8*18mm, the tablet weight is 800mg, and the temperature of the material before tableting is kept below 25°C.
[0035] Set the air inlet temperature to 70°C, and use 12% coati...
Embodiment 2
[0038] Prepare 11% hypromellose aqueous solution as adhesive. Weigh 600g of lipoic acid and 115g of microcrystalline cellulose, place them in a wet granulator, turn on the stirring blade at 400rpm, and the shear knife at 2000rpm, mix for 2 minutes, spray 190g of binder solution into granules, and pass through an 18-mesh sieve. Granules were dried in a fluidized bed at 40°C for 30 minutes, and granulated with a 18-mesh sieve.
[0039] Put the dry granules in a mixer, add 40.1g of low-substituted hydroxypropyl cellulose and 9g of magnesium stearate and mix for 5 minutes, then granulate with a dry granulator, set the pressure at 3MPa, the speed of the roller is 10rpm, and the speed of the granulator is 70rpm , 18 mesh sieve granulation.
[0040]Add 15g of magnesium stearate, mix for 5 minutes, use special-shaped punched tablets with a diameter of 8*18mm, and the weight of the tablet is 800mg. Before tableting, the temperature of the material is lowered to below 25°C.
[0041] ...
Embodiment 3
[0044] Weigh 600g of lipoic acid, 60g of low-substituted hydroxypropyl cellulose, and 25g of hydroxypropyl cellulose, place them in a wet granulator, turn on the stirring blade at 400rpm, and the shearing knife at 2000rpm, mix for 1 minute, and use 50% ethanol solution as a wetting agent, spray an appropriate amount of wetting agent solution to granulate, pass through a 16-mesh sieve for granulation, dry in a fluidized bed at 35°C for 40 minutes, and granulate with a 18-mesh sieve.
[0045] Put the dry granules in a mixer, add 96g of low-substituted hydroxypropyl cellulose and 9g of magnesium stearate and mix for 5 minutes, then granulate with a dry granulator, set the pressure at 3MPa, the speed of the pressing roller is 10rpm, and the speed of the granulator is 80rpm. 18 mesh sieve for granulation.
[0046] Add 10g of magnesium stearate, mix for 5 minutes, use special-shaped punched tablets with a diameter of 8*18mm, and the weight of the tablet is 800mg. Before tableting, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com