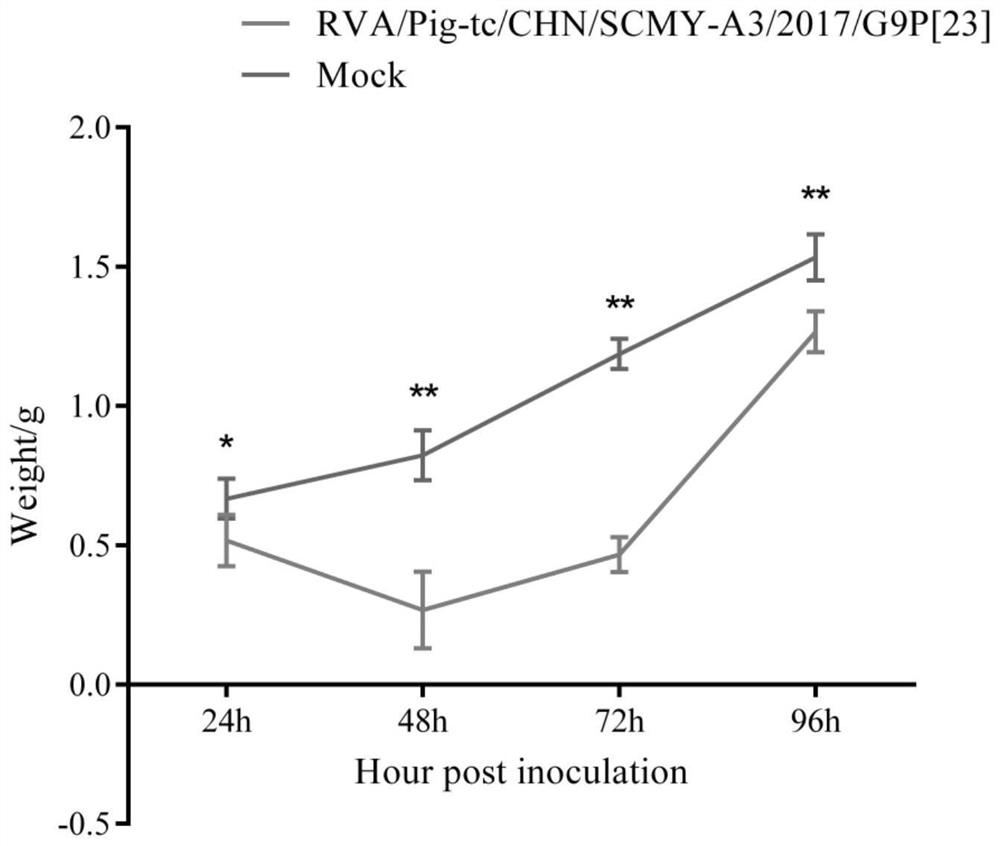
Porcine rotavirus strain, inactivated vaccine prepared from porcine rotavirus strain and application of porcine rotavirus strain
A porcine rotavirus, rotavirus technology, applied in the direction of viruses, vaccines, antiviral agents, etc., can solve the problems of low antibody level and weak immunity, and achieve the effect of good safety and good commercial development prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Isolation and pathogenicity identification of porcine rotavirus strain of embodiment 1
[0041] Isolation of porcine rotavirus strains
[0042] Take an appropriate amount of stool sample and PBS buffer, mix and vortex according to the ratio of 1:10, freeze and thaw repeatedly three times, absorb the supernatant after centrifugation, filter and sterilize with a filter with a diameter of 0.22 μm. Treated samples were treated with trypsin (TPCK trypsin) at a final concentration of 30 μg / mL at 37°C, 5% CO 2 Under the conditions of 1h, inoculated in a single layer of MA-104 cells, placed in 37 ° C, 5% CO 2 Cultivate in an incubator, observe the state of the cells, and place the cell culture in a -80°C refrigerator for rapid freezing until the cytopathic pathology (CPE) reaches about 80%. After repeated freezing and thawing for 3 times, the supernatant virus liquid is harvested by centrifugation. The virus liquid was purified three times by plaque technique. The isolated s...
Embodiment 2
[0061] The preparation of embodiment 2 porcine rotavirus strain inactivated vaccines
[0062] 1. Method
[0063] (1) Preparation of porcine G9 RVA virus liquid
[0064] First, the purified A3 isolate was treated with trypsin (TPCK trypsin) at a final concentration of 30 μg / mL at 37°C, 5% CO 2 Under the condition of 1h, inoculated in MA-104 cells, 37 ℃, 5% CO 2 Incubate in the incubator for 1 hour, shake once every 20 minutes; discard the suspension after incubation, add 35-40mL maintenance solution (DMEM containing 1% double antibody) at the same time, place at 37°C, 5% CO 2 Cultivate in an incubator; observe the cell state every day until the cell pathological change (CPE) reaches about 80%, place the cell culture in a -80°C refrigerator to freeze rapidly, and after repeated freezing and thawing for 3 times, centrifuge to harvest the supernatant virus liquid and multiply the virus liquid to 100mL for later use.
[0065] (2) Condition optimization of inactivator
[0066] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com