Preparation method of high-purity hydrofluoric acid
A hydrofluoric acid, pure-grade technology, applied in hydrogen fluoride, fluorine/hydrogen fluoride, etc., can solve the problems of reducing the purity of hydrofluoric acid preparation, and achieve the effects of speeding up cooling efficiency, improving reaction efficiency, and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
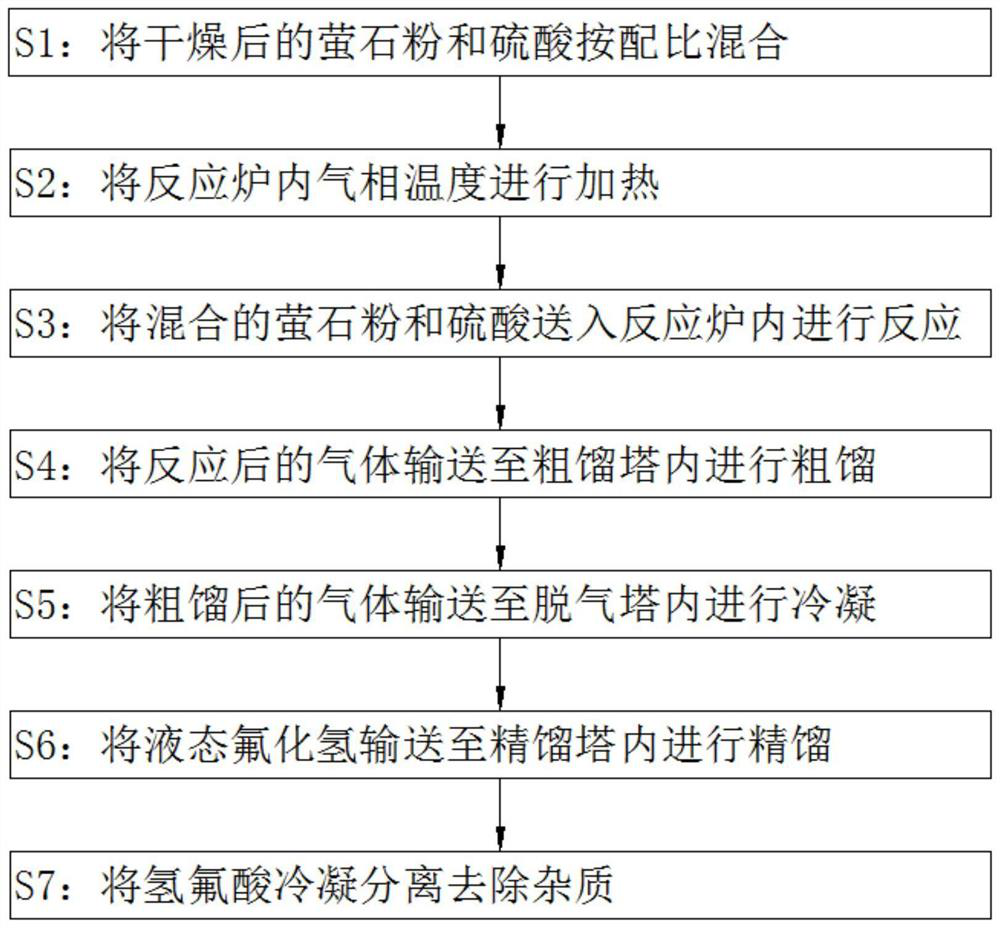
[0030] refer to figure 1 , a preparation method of high-purity grade hydrofluoric acid, comprising the following steps:
[0031] S1: Mix the dried fluorite powder and sulfuric acid according to the ratio;
[0032] S2: heating the reaction furnace;
[0033] S3: Send the mixed fluorite powder and sulfuric acid into the reaction furnace for reaction;
[0034] S4: transporting the reacted gas to the crude distillation tower for crude distillation;
[0035] S5: Transport the crudely distilled gas to the degassing tower for condensation;
[0036] S6: Transport the liquid hydrogen fluoride to the rectification tower for rectification;
[0037] S7: Condensing and separating the hydrofluoric acid to remove impurities.
[0038] In this embodiment, in S1, the fluorite powder is fully dried by the drying mechanism, and then the dried fluorite powder and sulfuric acid are weighed by a weighing instrument, and the fluorite powder and sulfuric acid are mixed by the stirring mechanism. ...
Embodiment 2
[0048] refer to figure 1 , a preparation method of high-purity grade hydrofluoric acid, comprising the following steps:
[0049] S1: Mix the dried fluorite powder and sulfuric acid according to the ratio;
[0050] S2: heating the reaction furnace;
[0051] S3: Send the mixed fluorite powder and sulfuric acid into the reaction furnace for reaction;
[0052] S4: transporting the reacted gas to the crude distillation tower for crude distillation;
[0053] S5: Transport the crudely distilled gas to the degassing tower for condensation;
[0054] S6: Transport the liquid hydrogen fluoride to the rectification tower for rectification;
[0055] S7: Condensing and separating the hydrofluoric acid to remove impurities.
[0056] In this embodiment, in S1, the fluorite powder is fully dried by the drying mechanism, and then the dried fluorite powder and sulfuric acid are weighed by a weighing instrument, and the fluorite powder and sulfuric acid are mixed by the stirring mechanism. Ac...
Embodiment 3
[0066] refer to figure 1 , a preparation method of high-purity grade hydrofluoric acid, comprising the following steps:
[0067] S1: Mix the dried fluorite powder and sulfuric acid according to the ratio;
[0068] S2: heating the gas phase temperature in the reaction furnace;
[0069] S3: Send the mixed fluorite powder and sulfuric acid into the reaction furnace for reaction;
[0070] S4: transporting the reacted gas to the crude distillation tower for crude distillation;
[0071] S5: Transport the crudely distilled gas to the degassing tower for condensation;
[0072] S6: Transport the liquid hydrogen fluoride to the rectification tower for rectification;
[0073] S7: Condensing and separating the hydrofluoric acid to remove impurities.
[0074] In this embodiment, in S1, the fluorite powder is fully dried by the drying mechanism, and then the dried fluorite powder and sulfuric acid are weighed by a weighing instrument, and the fluorite powder and sulfuric acid are mixed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com