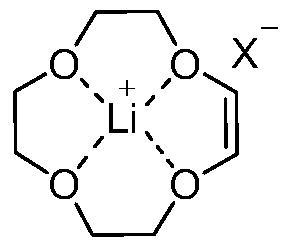
(Z)-1, 4, 7, 10-tetraoxocyclododecane-8-alkene lithium salt complex, preparation method and application thereof
A technology of tetraoxcyclododecane and tetraoxahexadecane is applied in the field of synthesizing crown ether compounds, which can solve the problems of difficult product purification, high risk of raw materials, low product yield, etc. The effect of labor cost, high reaction selectivity and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The present invention also provides a preparation method of (Z)-1,4,7,10-tetraoxocyclododecane-8-ene lithium salt complex, which comprises the following steps:
[0053]
[0054] Using compound R as a raw material, react with compound lithium salt LiX in the first reaction solvent, under the catalysis of ring-closing metathesis reaction catalyst and the protection of inert gas, under the condition of temperature range of -20 ~ 150 ℃, the reaction is complete The compound (Z)-1,4,7,10-tetraoxocyclododecane-8-ene lithium salt complex was obtained;
[0055] Wherein, R is selected from (2E,14E)-4,7,10,13-tetraoxahexadecane-2,14-diene, 3,6,9,12-tetraoxatetradecane-1, 13-diene, (2Z,14Z)-4,7,10,13-tetraoxahexadecane-2,14-diene, 4,7,10,13-tetraoxahexadecane-2, 14-diene, 5,8,11,14-tetraoxaoctadecane-3,15-diene, (3E,15E)-5,8,11,14-tetraoxaoctadecane-3, 15-diene, (3Z,15Z)-5,8,11,14-tetraoxaoctadecane-3,15-diene;
[0056] X is selected from fluorine, chlorine, bromine, iodine,...
Embodiment 1
[0076]
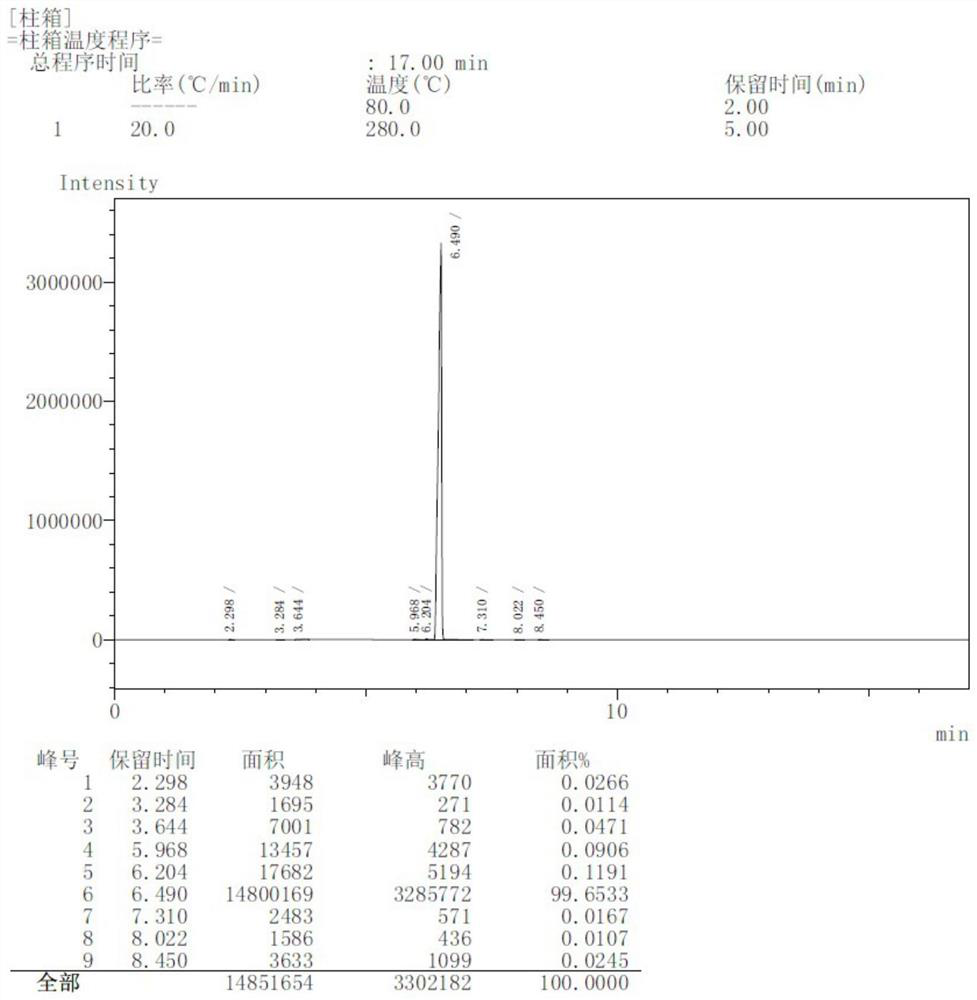
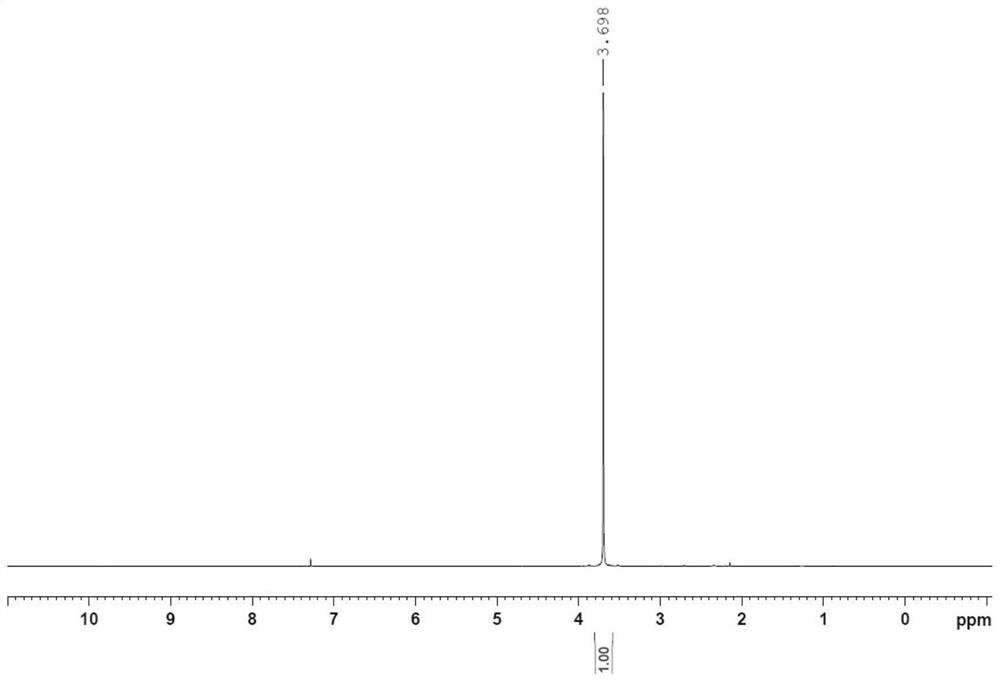
[0077] Step (a): Preparation of formula 3 compound (Z)-1,4,7,10-tetraoxocyclododecane-8-ene lithium chloride complex
[0078] Maintaining a slight positive pressure of nitrogen, add 60L ethyl acetate and 1.28kg formula 2 compound lithium chloride to the 100L reaction kettle successively, stir well; then add 1g GRELA2 generation catalyst to it, stir well; Add 6.91kg of the compound of formula 1 (2E,14E)-4,7,10,13-tetraoxahexadecane-2,14-diene dropwise into the reaction solution, control the rate of addition, drop it in 3 hours, stir well, The gas generated during the reaction is introduced into the potassium permanganate aqueous solution, which will produce olefin gas, but the danger is much lower than hydrogen gas, which can be absorbed by the potassium permanganate aqueous solution, while the hydrogen gas produced by the prior art cannot be absorbed . When the color of the potassium permanganate aqueous solution starts to lighten, it is necessary to add potassium...
Embodiment 2
[0093]
[0094] Step (a): Preparation of formula 3 compound (Z)-1,4,7,10-tetraoxocyclododecane-8-ene lithium chloride complex
[0095] Maintaining a slight positive pressure of nitrogen, sequentially add 60L of ethyl acetate and 1.28kg of formula 2 compound lithium chloride into the 100L reactor, and stir well; then add 1g of GRELA 2nd generation catalyst to it, stir well; the temperature of the reaction solution is raised to 50°C, Add 6.07kg formula 1 compound 3,6,9,12-tetraoxatetradecane-1,13-diene dropwise in the reaction solution, control the rate of addition, drop it in 3 hours, stir well, the The gas is introduced into the potassium permanganate aqueous solution, and when the color of the potassium permanganate aqueous solution starts to become lighter, potassium permanganate needs to be added thereto. The reaction solution was kept at 50° C. to continue the reaction for 8 hours, and the reaction was completed.
[0096] The reaction solution was naturally cooled to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com