Polyfluoride red-light-emitting material for solid-state lighting LED and preparation method and application of polyfluoride red-light-emitting material
A solid-state lighting and fluoride technology, applied in the direction of luminescent materials, chemical instruments and methods, electrical components, etc., can solve problems such as synthesis methods and emission efficiency constraints, and achieve good stability, high color reproduction, and high light efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take 15 ml of hydrofluoric acid from the polytetrafluoroate beaker with a pipette, adding a stirrer, and sequentially add 0.252 gram of sodium sodium sodium fluoride, 0.156 grams of lithium flucture and 0.555 g of dioxide, and stirring at a constant mixing. After 10 min, the polytetrafluoroethylene beaker was transferred to an oil bath of 130 ° C, and 0.012 grams of potassium manganganate was added after 3 hours, and then the beaker was removed from the oil bath and cooled from the oil bath. Ethanol. Wash the resulting solid, and finally dry 24 h in a vacuum drying box, resulting in the light pink powder, which is NA 3 Li 3 In 2 Fly 12 : MN 4+ Red light material.
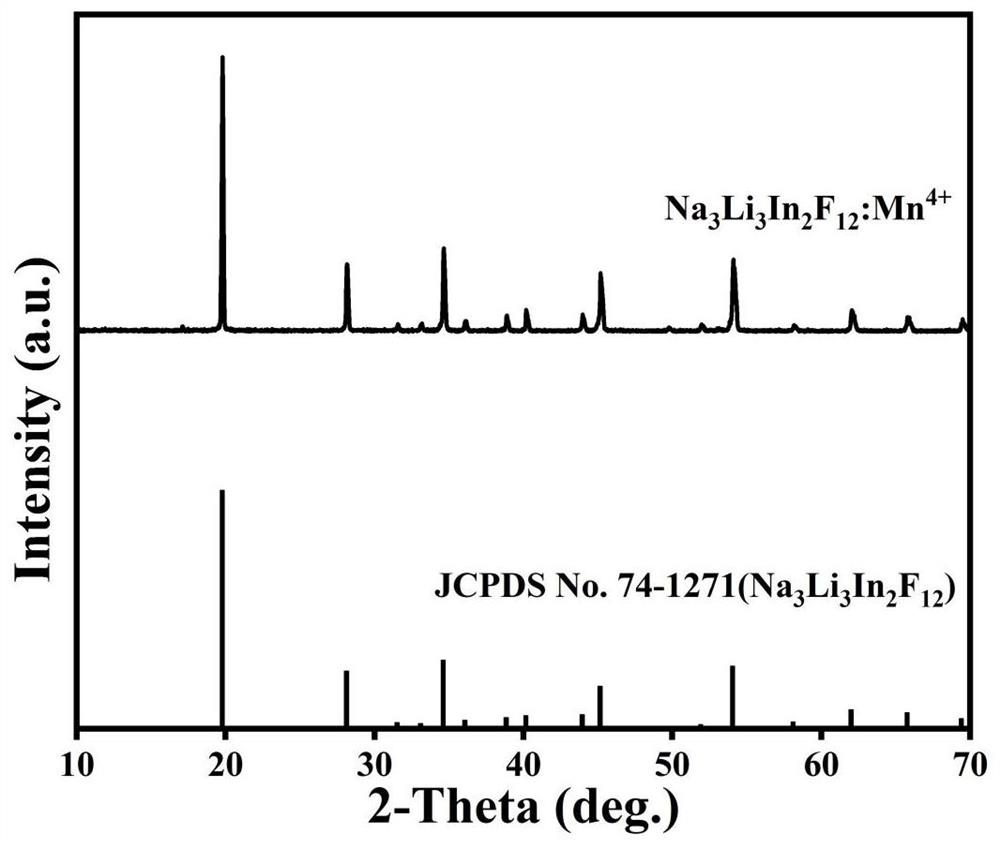
[0030] XRD diffraction map of the red light material figure 1 As shown, the diffraction peak of the sample is exactly the same as the standard card JCPDS74-1271, and no hybrid or peak offset is observed.
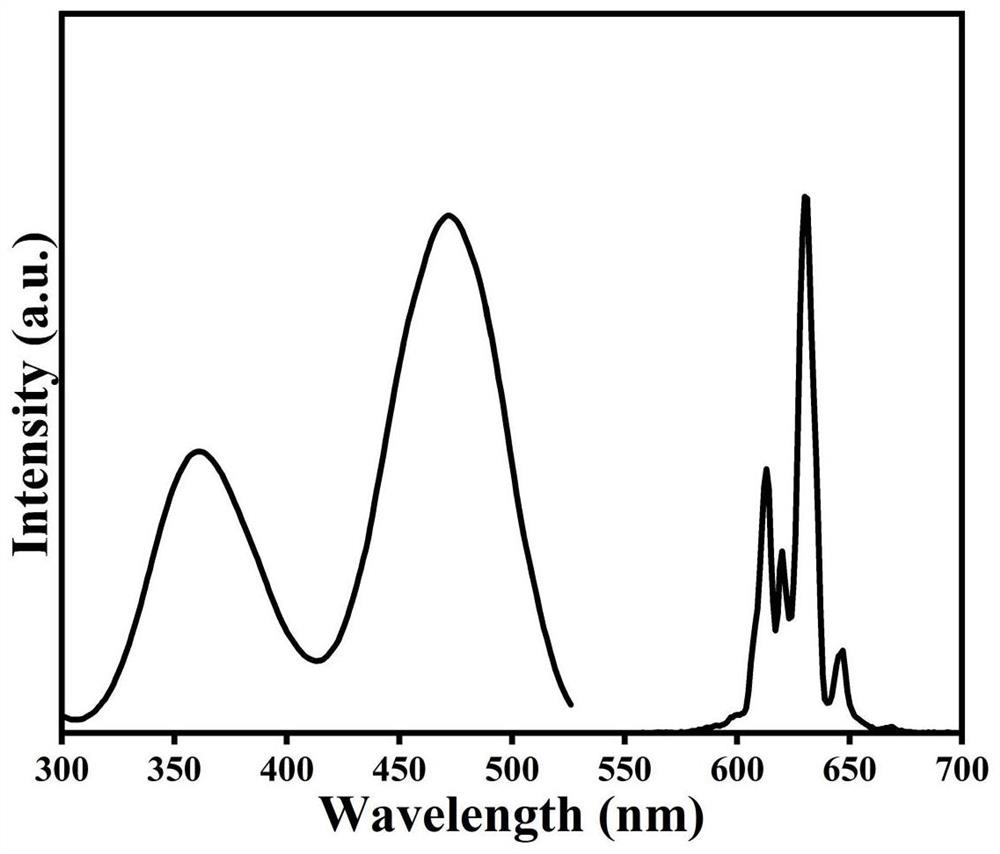
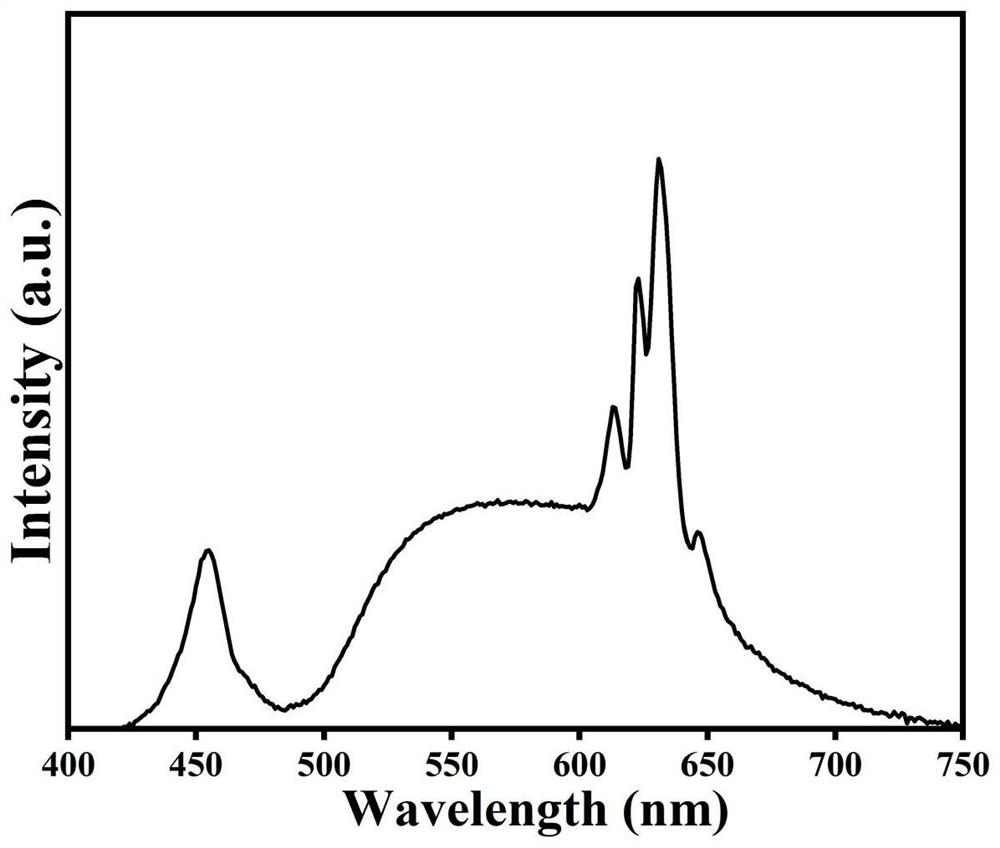
[0031] Attach figure 2 For the excitation spectrum and emission spectrum of the red light material at room t...
Embodiment 2
[0036] Take 15 ml of hydrofluoric acid from the polytetrafluoroethylene beaker with a pipette, adding a stirrer, sequentially added 0.317 grams of sodium carbonate, 0.206 g of lithium nitrate and 0.555 grams of sodium nitrate and 0.555 g of dioxide, and stirred at a constant mixing 10min The polytetrafluoroethylene beaker was transferred to an oil bath of 130 ° C, and 0.012 grams of potassium manganganate was added after 3 h, and then the beaker was removed from the oil bath and washed with anhydrous ethanol. Solid, finally dried in a vacuum drying box for 24 h, the obtained light pink powder is NA 3 Li 3 In 2 Fly 12 : MN 4+ Red light material.
Embodiment 3
[0038] Take 15 ml of hydrofluoric acid from the polytetrafluoroate beaker with a pipette, adding a stirrer, and sequentially add 0.252 gram of sodium sodium sodium fluoride, 0.156 grams of lithium flucture and 0.555 g of dioxide, and stirring at a constant mixing. After 10 min, the polytetrafluoroethylene beaker was transferred to an oil bath of 120 ° C, and 0.012 grams of potassium manganganate was added after 3 hours, and then the beaker was taken out from the oil bath and cooled to room temperature. Ethanol. Wash the resulting solid, and finally dried in a vacuum drying tank for 24 h, the obtained light pink powder is NA 3 Li 3 In 2 Fly 12 : MN 4+ Red light material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Color temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com