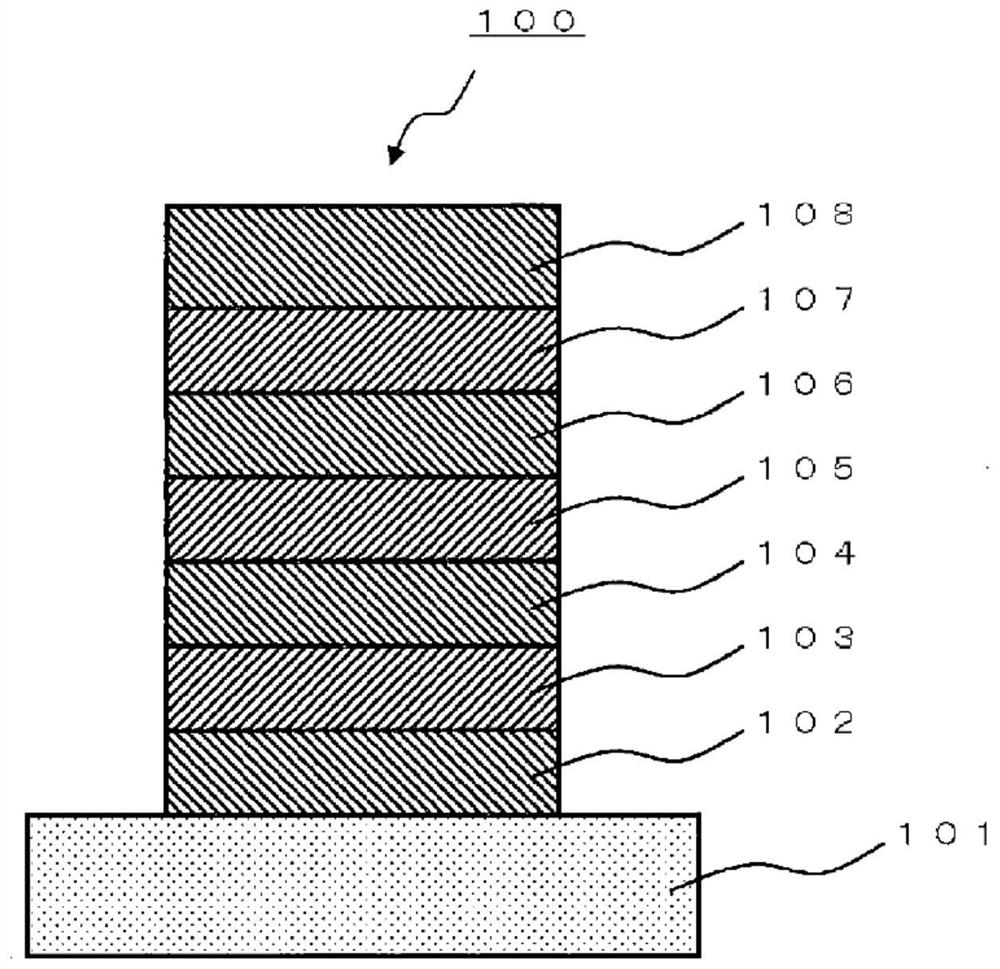
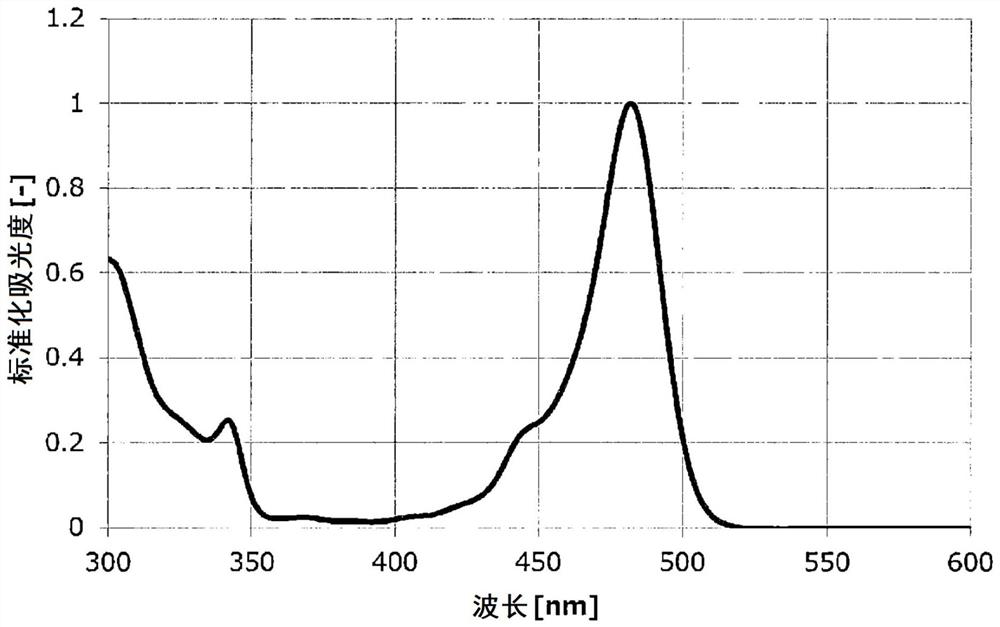
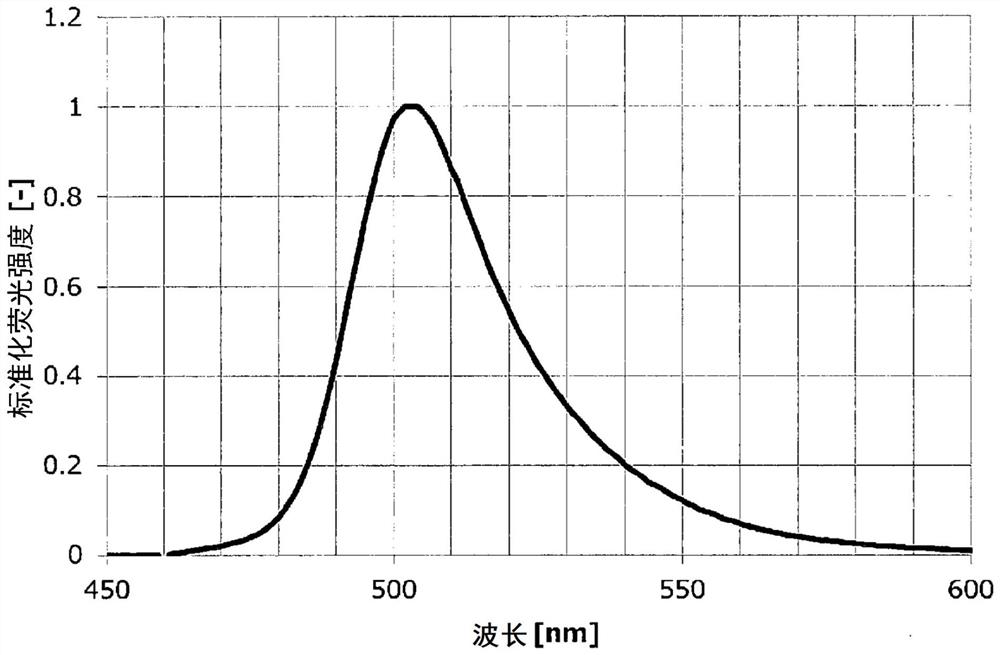
Polycyclic aromatic compound
A polycyclic aromatic compound technology, applied in the field of display devices and lighting devices, can solve the problems of insufficient redox stability, insufficient lifetime, and low triplet excitation energy of small aromatic rings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0787] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to them. First, a synthesis example of a polycyclic aromatic compound will be described below.
Synthetic example (1
[0789] Compound (1-230): 7,10-bis(trimethylphenyl)-2,15,18-trimethyl-5,12-dioxa-8b-aza-16b,19b-diboronanthracene[ Synthesis of 1,9-ab]benzo[j]perylene
[0790]
[0791] Under a nitrogen atmosphere, a mixture of 1-bromo-3-chloro-5-fluorobenzene (compound I-A, 14.7g, 70mmol), p-cresol (compound I-B, 7.95g, 74mmol), potassium carbonate (14.5g, 105mmol) ) and N-methylpyrrolidone (NMP) 500ml flask was heated to 150°C and stirred for 15 hours. The reaction solution was cooled to room temperature, filtered through a short column (eluent: toluene) using Florisil, and extracted with hydrochloric acid and toluene to obtain 1-bromo-3-chloro-5-(p-tolyloxy ) Benzene (Compound I-C) (18.4 g, 88% yield).
[0792]
[0793] The structure of the obtained compound was confirmed by NMR measurement.
[0794] 1 H-NMR (400MHz, CDCl 3 ): δ=2.35(s,3H), 6.86-6.98(m,4H), 7.13-7.18(m,3H).
[0795] Under nitrogen atmosphere, the compound I-C (13.1g, 44mmol), p-toluidine (compound I-D, 2.15g, 20...
Synthetic example (2
[0809] Compound (1-28): 7,10-di(trimethylphenyl)-2,15,18-trimethyl-5,12-di-p-tolyl-5,12H-dihydro-5,8b,12- Synthesis of triaza-16b,19b-diboronanthracene[1,9-ab]benzo[j]perylene
[0810]
[0811] Under a nitrogen atmosphere, 1,3-dibromo-5-chlorobenzene (compound I-H, 5.41g, 20mmol), di-p-toluidine (compound I-I, 3.95g, 20mmol), Pd 2 (dba) 3 (0.183mg, 0.2mmol), BINAP (0.249mg, 0.4mmol), tBuONa (2.12g, 22mmol) and toluene (100ml) were heated to 80°C and stirred for 40 hours. The reaction solution was cooled to room temperature, filtered using a short column filled with Florisil (eluent: toluene), and then filtered using a short column filled with silica gel (eluent: hexane), thereby obtaining 3-bromo -5-Chloro-N,N-di(p-tolyl)amine (compound I-J) (7.29 g, yield 50%).
[0812]
[0813] The structure of the obtained compound was confirmed by NMR measurement.
[0814] 1 H-NMR (400MHz, CDCl 3): δ=2.33(s,6H), 6.82(s,1H), 6.94-7.00(m,5H), 7.10-7.12(s,4H).
[0815] Under nitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com