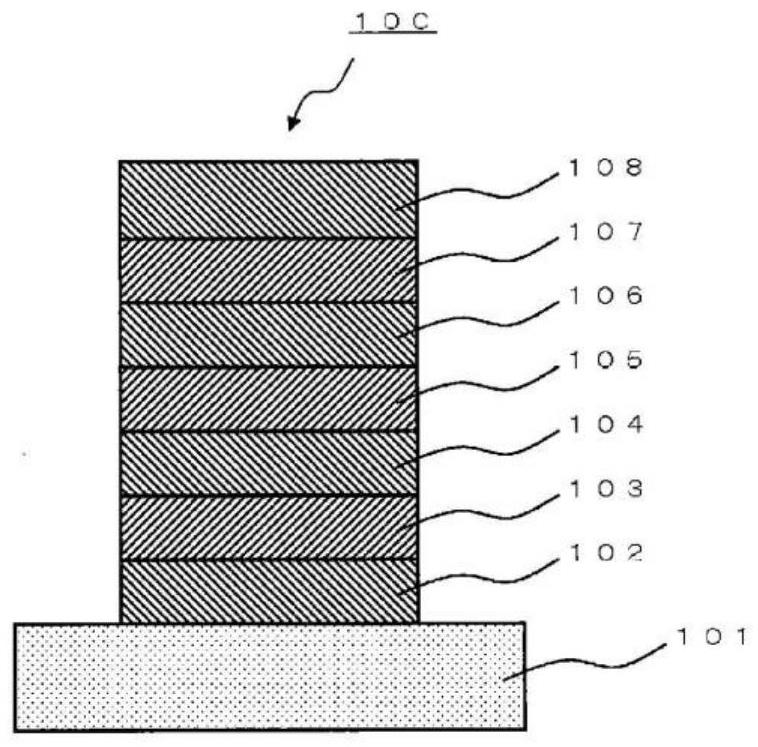
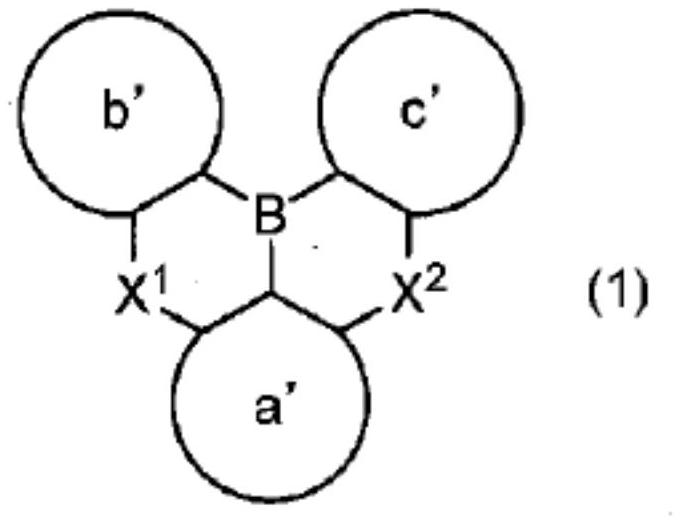
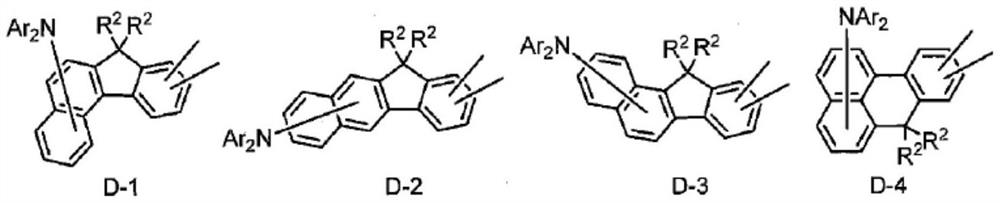
Polycyclic aromatic compound
A polycyclic aromatic compound technology, applied in the field of display devices and lighting devices, can solve the problems of insufficient life, unsuitable main material, insufficient redox stability of aromatic epoxies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[1437] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to these Examples. First, a synthesis example of a polycyclic aromatic compound will be described below.
Synthetic example (1
[1439] Synthesis of compound (A-1)
[1440] 17,17-Dimethyl-N,N-diphenyl-17H-5,9-dioxa-18b-borabenzo[6,7]indeno[1,2-b]naphtho[1 ,2,3-fg]anthracen-15-amine
[1441] Synthesis of compound (A-311)
[1442] 17,17-Dimethyl-N,N-diphenyl-17H-5,9-dioxa-18b-borabenzo[de]naphtho[3,2,1-op]pentacene- 13-amine
[1443] [chem 399]
[1444]
[1445]Under a nitrogen atmosphere, put methyl 2-hydroxyl-4-methoxybenzoate (125g) and pyridine (825ml) into a flask, and after cooling in an ice bath, add trifluoroethylene dropwise in an ice bath Methanesulfonic anhydride (387.2 g). Then, after stirring at room temperature for 2 hours, water was added to stop the reaction. After adding toluene for liquid separation, purification was carried out with a silica gel short path column (eluent: toluene) to obtain methyl 4-methoxy-2-(((trifluoromethyl)sulfonyl)oxy)benzene Formate (216 g).
[1446] [chem 400]
[1447]
[1448] Under nitrogen atmosphere, 4-bromo-N,N-diphenylnaphthalene-1-amine (17...
Synthetic example (2
[1483] Synthesis of compound (A-81)
[1484] 17,17-Dimethyl-N,N,5-triphenyl-5H,17H-9-oxa-5-aza-18b-borabenzo[6,7]indeno[1,2- b]naphtho[1,2,3-fg]anthracene-15-amine
[1485] Synthesis of compound (A-341)
[1486] 17,17-Dimethyl-N,N,5-triphenyl-5H,17H-9-oxa-5-aza-18b-borabenzo[de]naphtho[3,2,1- op]pentacene-13-amine
[1487] [chem 409]
[1488]
[1489] Under nitrogen atmosphere, 5-(diphenylamino)-7,7-dimethyl-7H-benzo[c]fluoren-10-ol and 3-(diphenylamino)-7,7 -Dimethyl-7H-benzo[de]anthracene-10-ol mixture (40g), 1-bromo-2-chloro-3-fluorobenzene (25.5g), potassium carbonate (32.3g) and NMP (200ml ) flask was heated and stirred at reflux temperature for 5 hours. After the reaction was stopped, the reaction solution was cooled to room temperature, and water and toluene were added for liquid separation. The solvent in the organic layer was distilled off under reduced pressure, and then purified with a short silica gel column (eluent: toluene). Secondly, use Solmix (Solmi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com