Process for cleanly producing chromic anhydride crystal by sodium nitrate circulation method
A clean production, chromic anhydride technology, applied in the directions of chromium trioxide, chromium oxide/hydrate, etc., can solve the problems of serious environmental pollution, high production cost, low effective metal recovery rate, etc., and achieve lower reaction temperature and faster production speed. , the effect of increasing the sales price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
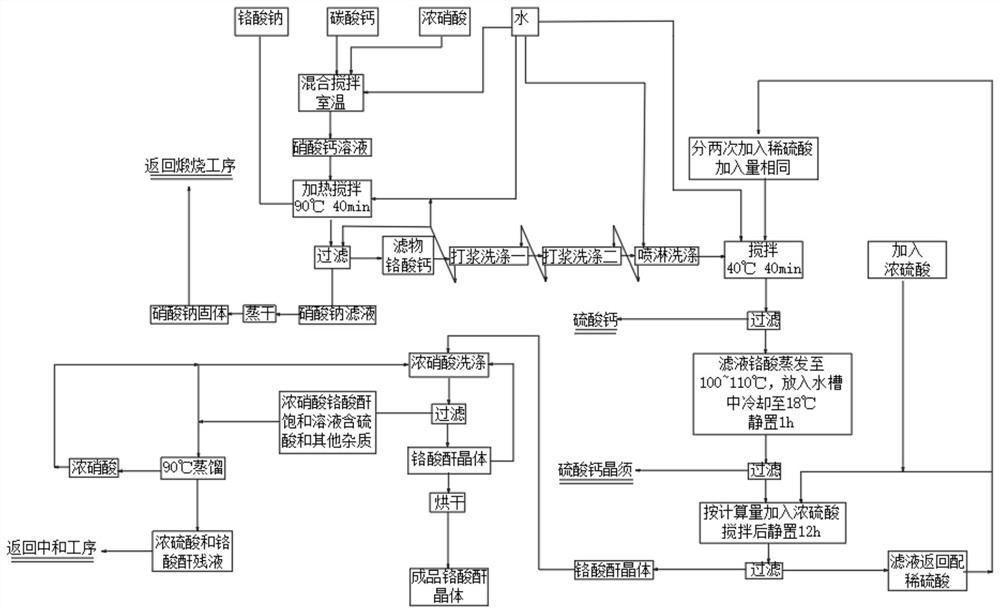
[0042] Accurately weigh (analytical pure calcium carbonate, content is 99.0%) CaCO3 43g, add 114ml of distilled water into the calcium carbonate at room temperature, after fully stirring, gradually add (analytical pure concentrated nitric acid, content is 65~68%) HNO3 57ml , The solution was reacted under stirring until clear and transparent. Then add (analytical pure sodium chromate, content is 99.0%) Na2CrO4 4H2O100g, in a water bath, under strong stirring, stir at 90°C for 40 minutes, then filter at 80°C, the filter is calcium chromate Crystals, the filtrate is sodium nitrate solution. After the sodium nitrate solution is concentrated and crystallized by evaporation (return to another sodium chromate making process for recycling).
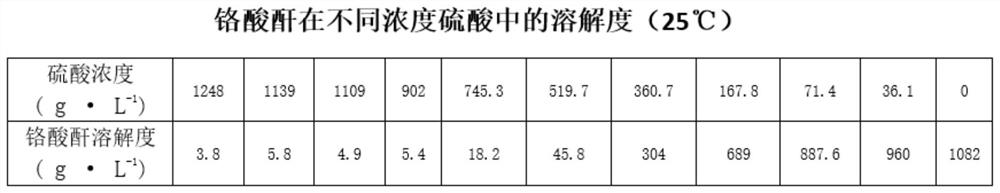
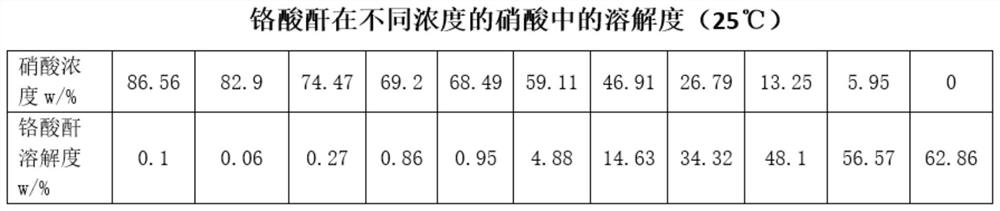
[0043] Add 100ml of distilled water to the calcium chromate crystals obtained above, stir in a water bath at a constant temperature of 40°C for 10 minutes, then gradually add 60% dilute sulfuric acid (dilute sulfuric acid is prepared by 95-98% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com