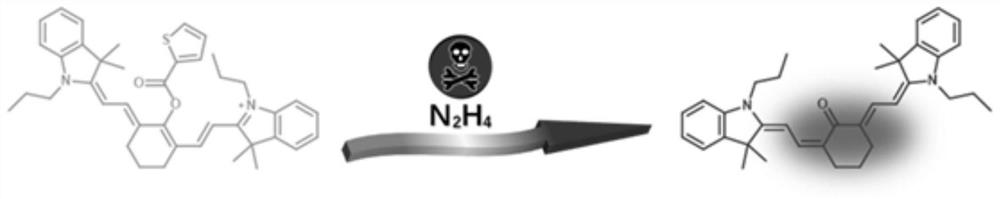
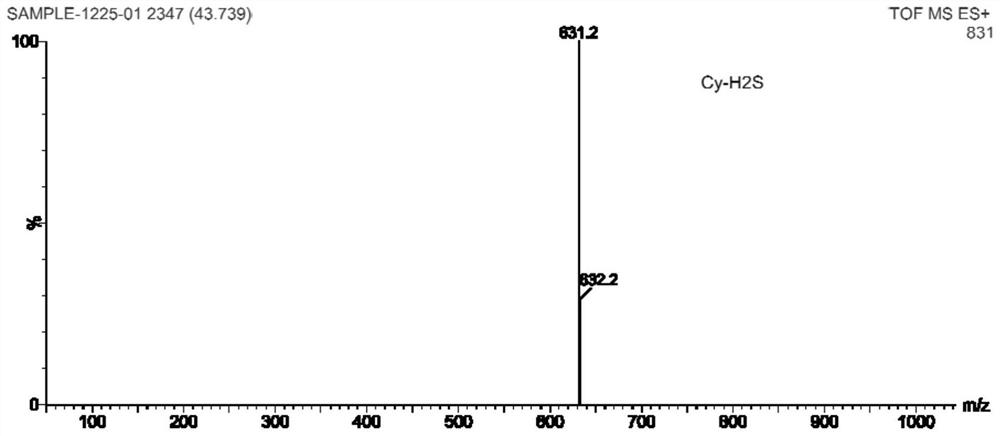
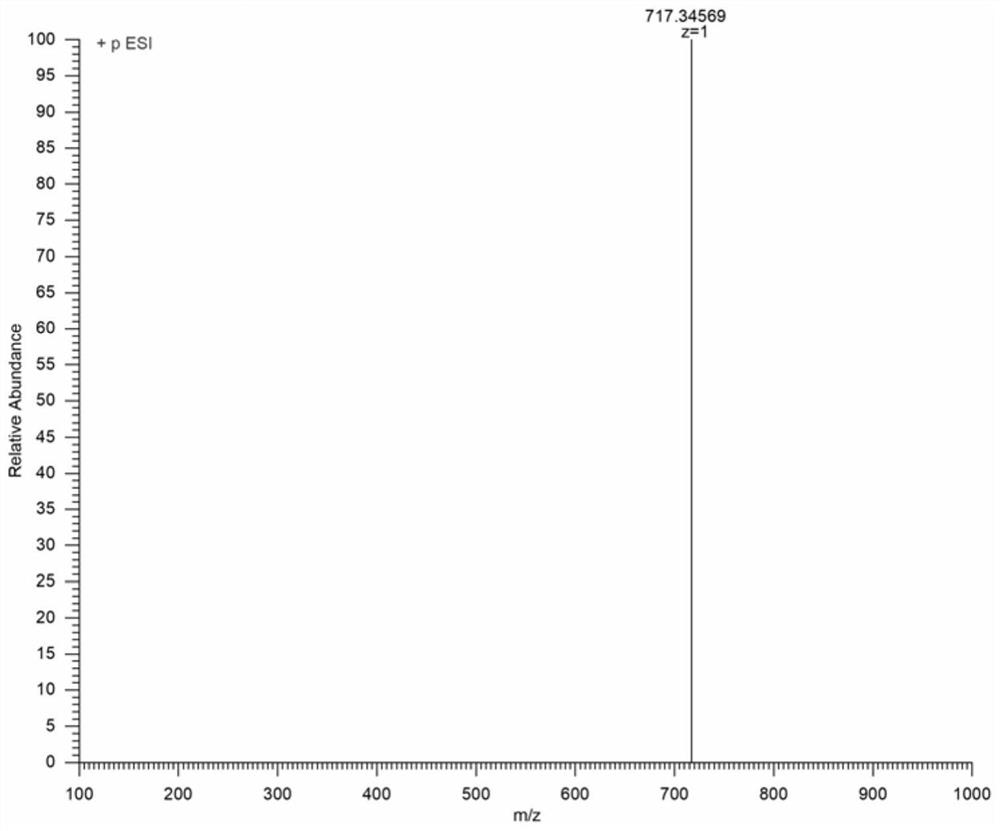
Near-infrared cyanine colorimetric fluorescent probe and preparation method and application thereof
A fluorescent probe, near-infrared technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of scarcity of near-infrared fluorescent probes, poor anti-interference ability, and homeostasis destruction, and achieve the realization of Tracer imaging detection, strong anti-interference ability, good sensing properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 Synthesis of near-infrared cyanine colorimetric fluorescent probe VI
[0076] The fluorescent probe VI of the present embodiment corresponds to the R of the compound of formula (I) 1 for hydrogen, R 2 Is propyl, n is 1.
[0077] The reaction formula for synthesizing near-infrared cyanine-like colorimetric fluorescent probe VI is as follows (A):
[0078]
[0079] Concrete steps for synthesizing near-infrared cyanine-like colorimetric fluorescent probe VI:
[0080] (1) Synthesis of intermediate V: Dissolve IV and sodium acetate in anhydrous N,N-dimethylformamide (15mL) at a molar ratio of 1:5 to 1:2, under an argon atmosphere at 75 to 90°C Heating for 2 to 6 hours. After the mixture was cooled to room temperature, it was filtered, and the resulting solution was concentrated under reduced pressure to obtain an oily crude product. Finally, separation and purification by silica gel column chromatography (dichloromethane as eluent) gave the pure intermediate...
Embodiment 2
[0083] Example 2 Synthesis of near-infrared cyanine-like colorimetric fluorescent probe IX
[0084] Fluorescent probe IX of the present embodiment corresponds to the R of the compound of formula (I) 1 is a benzene ring, R 2 is propyl, n is 0.
[0085] The reaction formula for synthesizing near-infrared cyanine-like colorimetric fluorescent probe IX is as follows (B):
[0086]
[0087] Concrete steps for synthesizing near-infrared cyanine-like colorimetric fluorescent probe IX:
[0088] (1) Synthesis of intermediate VIII: Dissolve VII and sodium acetate in anhydrous N,N-dimethylformamide (15mL) at a molar ratio of 1:1 to 1:4, under an argon atmosphere at 70 to 90°C Heating for 1 to 6 hours. After the mixture was cooled to room temperature, it was filtered, and the resulting solution was concentrated under reduced pressure to obtain an oily crude product. Finally, separation and purification by silica gel column chromatography (dichloromethane as eluent) gave the pure in...
Embodiment 3
[0091] Example 3 Synthesis of near-infrared cyanine-like colorimetric fluorescent probe XII
[0092] The fluorescent probe XII of the present embodiment corresponds to the R of the compound of formula (I) 1 is chlorine, R 2 is a methyl group, and n is 1.
[0093] The reaction formula for synthesizing the near-infrared cyanine-like colorimetric fluorescent probe XII is as follows (C):
[0094]
[0095] Concrete steps for synthesizing near-infrared cyanine-like colorimetric fluorescent probe XII:
[0096] (1) Synthesis of intermediate XI: Dissolve X and sodium acetate in anhydrous N,N-dimethylformamide (15mL) at a molar ratio of 1:3 to 1:1.5, under an argon atmosphere at 75 to 100°C Heating for 2 to 5 hours. After the mixture was cooled to room temperature, it was filtered, and the resulting solution was concentrated under reduced pressure to obtain an oily crude product. Finally, separation and purification by silica gel column chromatography (dichloromethane as the elu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com