Synthesis method of double-arm intermediate LND1037 for antibody coupling medicine
A technology of LND1037 and antibody-drug coupling, which is applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., and can solve the problems of low synthesis yield of intermediate 6, difficult purification, and difficulty in scale-up production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
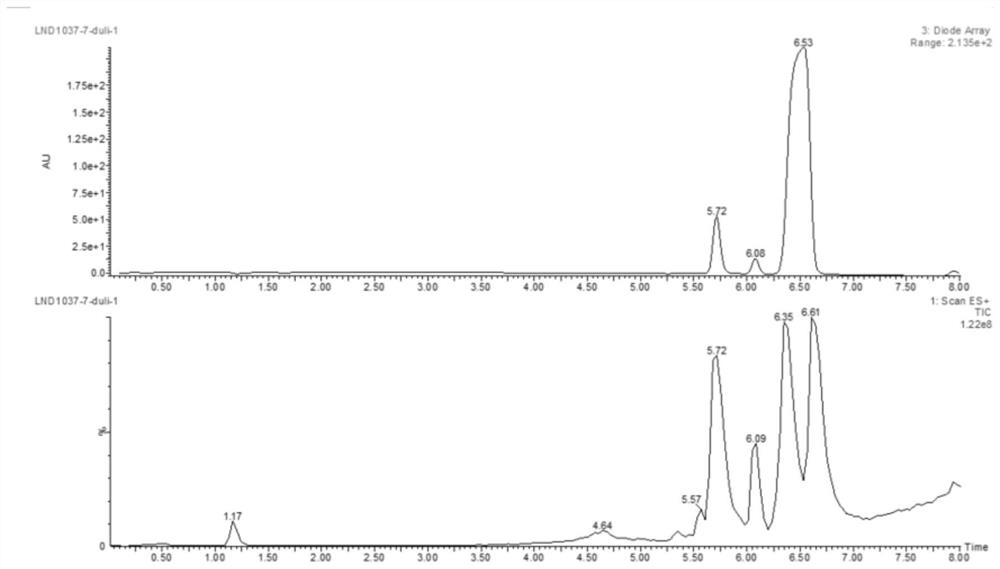
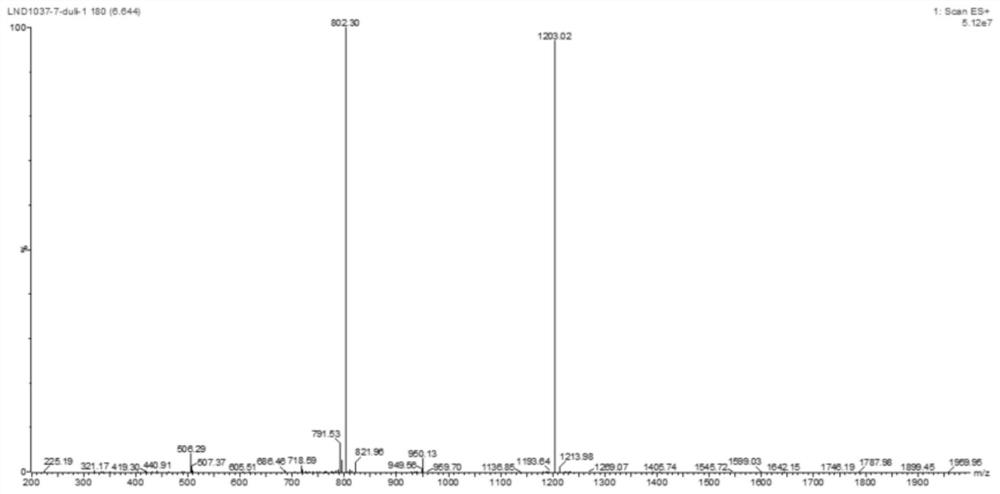
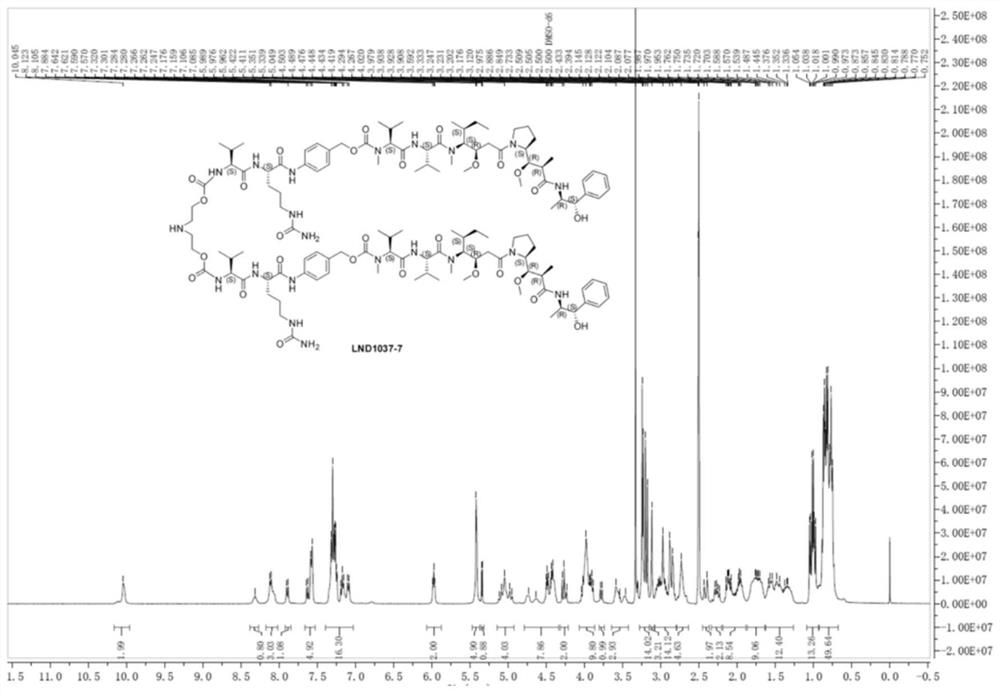
Image
Examples
Embodiment 1
[0055] This example provides a method for synthesizing the two-arm intermediate LND1037 for antibody-drug conjugates, including the following steps.
[0056] Synthesis of LND1037-1
[0057] Fmoc-ADE (100g, 0.306mol, 1eq) and DNPC (186g, 0.612mmol, 2eq) were dissolved in 1L DMF, DIPEA (3.92g, 30.4mmol, 0.1eq) was added, stirred at room temperature for 16 hours, TLC showed that the raw material reacted Complete, add 5L of 5% citric acid aqueous solution to the reaction solution, extract three times with 5L ethyl acetate respectively, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, and purify through a silica gel column. The mobile phase is ethyl acetate:petroleum ether=30: 70, to obtain LND1037-1 (167 g, yield 84%).
[0058] Synthesis of LND1037-4
[0059] BCNOH (100g, 0.666mol, 1eq) was dissolved in dichloromethane (2L), nitrogen was replaced twice, chlorosulfonyl isocyanate (94g, 0.666mol, 1eq) was added, and stirred at room temperature for 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com