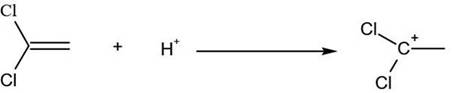Preparation method of 2, 4-dichloro-5-fluoroacetophenone
A technology of fluoroacetophenone and dichlorofluorobenzene, which is applied in the field of preparation of 2,4-dichloro-5-fluoroacetophenone, can solve the problems of difficult wastewater treatment, inability to reuse, complex components of hydrolyzed mother liquor, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
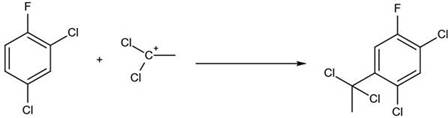
[0021] (1) Take 330g (2mol) of 2,4-dichlorofluorobenzene and add it to the reaction kettle with mechanical stirring, slowly pass in 0.73g (0.02mol) of hydrogen chloride, and put in 53.4g (0.4mol) of anhydrous aluminum chloride and 97g (1mol) of vinylidene chloride, tighten the lid of the kettle, start stirring and gradually heat up to 150°C, and keep the temperature for 2 hours;
[0022] (2) After the heat preservation is over, cool down to 75°C, use 1-5min to slowly open the exhaust valve, and the exhaust gas will be cooled through the condenser tube, and the cooled material is vinylidene chloride;
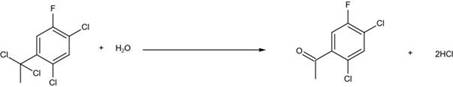
[0023] (3) Transfer the materials in the reaction kettle to a glass reaction bottle, raise the temperature to 130°C, slowly add an appropriate amount of water to keep the temperature at 130°C, and use it for 1-2 hours to slowly add dropwise until the temperature cannot be maintained, which means the hydrolysis reaction It is basically over, keep warm for 0.5 hours;
[0024] (4) ...
Embodiment 2
[0035] The difference between this implementation method and Example 1 is that in order to improve efficiency, no water layer is added after the hydrolysis reaction, and the internal standard is directly sampled to detect 2,4-dichlorofluorobenzene and 2,4-dichloro-5-fluorophenylethyl Ketone amount, its transformation rate is 41.5%, and selectivity is 81.8%.
Embodiment 3
[0037] The difference between this implementation method and Example 2 is that the Friedel-Crafts reaction temperature in step (1) is 130°C, the other steps are the same, the conversion rate is 40.7%, and the selectivity is 87.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com