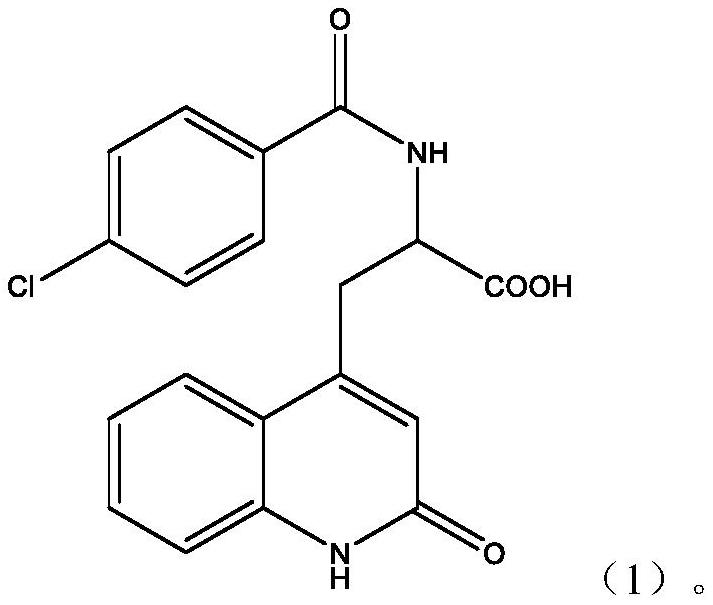
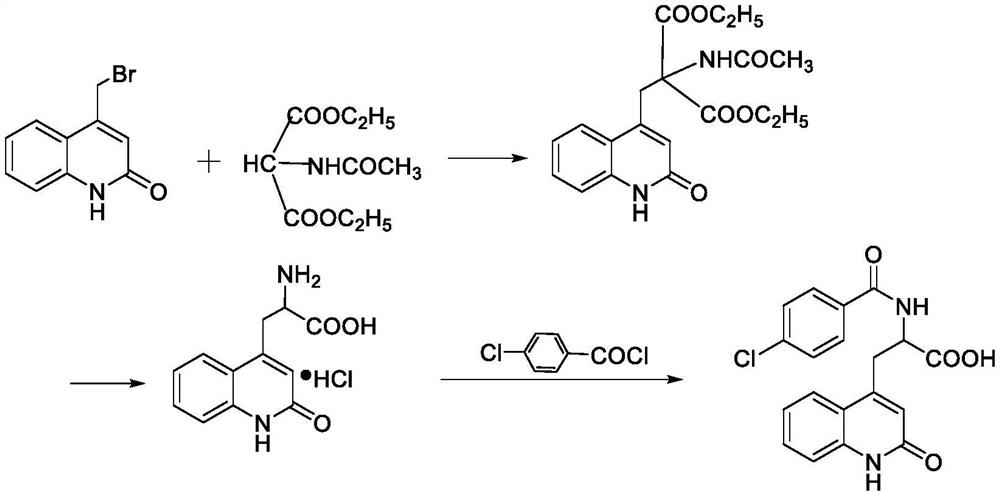
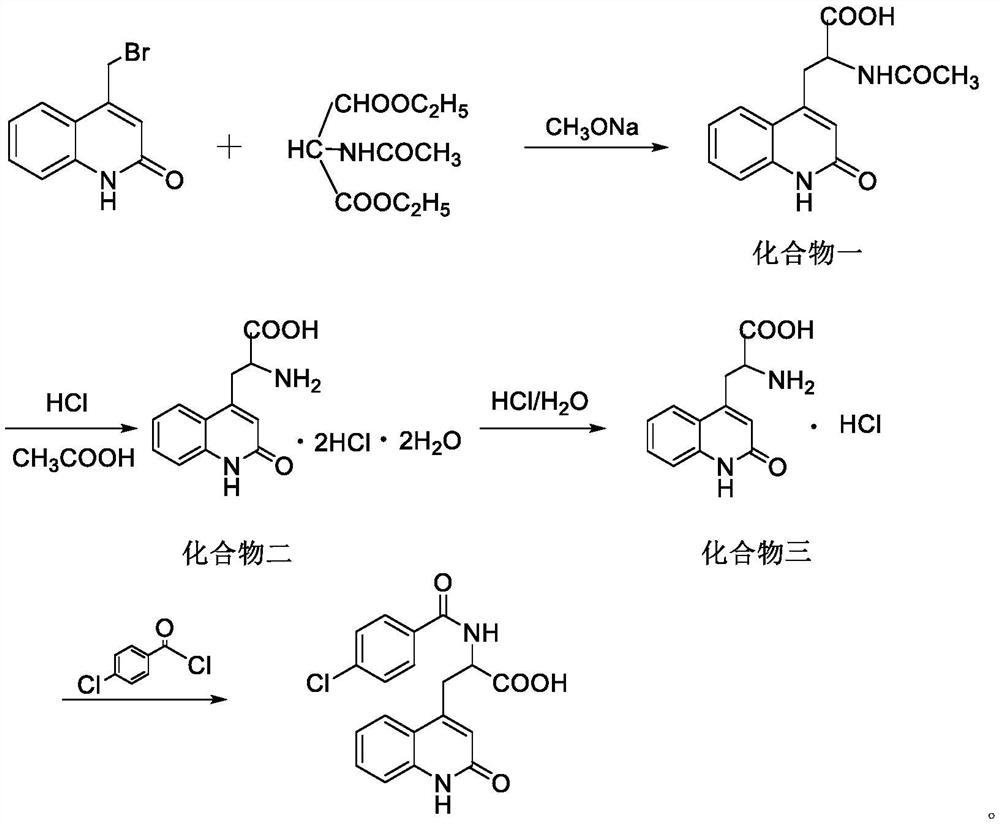
Preparation method of rebamipide bulk drug
A technology of rebamipide and raw material medicine, which is applied in the field of preparation of rebamipide raw material medicine, can solve problems such as unfavorable industrial production, unsatisfactory improvement of purity, and increased production cost, so as to facilitate industrial production, avoid crystallization and purification, The effect of reducing the content of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1: Preparation of crude product of rebamipide bulk drug: Add sodium hydroxide solution to a three-necked flask with thermometer, reflux tube, and stirrer, and at 0°C, 150r / min, add compound three and Dimethylacetamide, after stirring at constant temperature and speed for 10 minutes, slowly add the toluene solution of 4-chlorobenzoyl chloride dropwise, and control the temperature of the reaction system during the dropwise addition process to be less than 5°C. React at 3°C for 5 hours, then add methanol, heat up to 75°C, add dropwise glacial acetic acid under stirring, cool to 22°C after the glacial acetic acid is added completely, then filter, wash the filter cake with deionized water and then with methanol to obtain The crude product of rebamipide bulk drug, wherein the mass ratio of sodium hydroxide solution, compound three, dimethylacetamide, 4-chlorobenzoyl chloride toluene solution, methanol, and glacial acetic acid is 450:28:95 :380:17, and the mass concentr...
Embodiment 2
[0039] Step 1: Preparation of crude product of rebamipide bulk drug: Add sodium hydroxide solution to a three-necked flask with thermometer, reflux tube, and stirrer, and at 0°C, 150r / min, add compound three and Dimethylacetamide, after stirring at constant temperature and speed for 10 minutes, slowly add the toluene solution of 4-chlorobenzoyl chloride dropwise, and control the temperature of the reaction system during the dropwise addition process to be less than 5°C. React at 3°C for 5 hours, then add methanol, heat up to 75°C, add dropwise glacial acetic acid under stirring, cool to 22°C after the glacial acetic acid is added completely, then filter, wash the filter cake with deionized water and then with methanol to obtain The crude product of rebamipide raw material drug, wherein, the mass ratio of sodium hydroxide solution, compound three, dimethylacetamide, 4-chlorobenzoyl chloride toluene solution, methanol, and glacial acetic acid is 480:29:100 :390:18, and the mas...
Embodiment 3
[0048] Step 1: Preparation of crude product of rebamipide bulk drug: Add sodium hydroxide solution to a three-necked flask with thermometer, reflux tube, and stirrer, and at 0°C, 150r / min, add compound three and Dimethylacetamide, after stirring at constant temperature and speed for 10 minutes, slowly add the toluene solution of 4-chlorobenzoyl chloride dropwise, and control the temperature of the reaction system during the dropwise addition process to be less than 5°C. React at 3°C for 5 hours, then add methanol, heat up to 75°C, add dropwise glacial acetic acid under stirring, cool to 22°C after the glacial acetic acid is added completely, then filter, wash the filter cake with deionized water and then with methanol to obtain The crude product of rebamipide bulk drug, wherein, the mass ratio of sodium hydroxide solution, compound three, dimethylacetamide, 4-chlorobenzoyl chloride toluene solution, methanol, and glacial acetic acid is 600:30:110 :400:20, and the mass concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com