A kind of thioxanthone visible light initiator and its preparation method and application
A thioxanthone, visible light technology, used in organic chemistry and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of N,N-bis(benzyl)thioxanthone photoinitiator (TXNB for short) 2 )
[0028] 2-Aminothioxanthone (3.41g, 15.0mmol), benzyl chloride (3.89g, 30.75mmol), anhydrous potassium carbonate ( 4.67 g, 33.825 mmol), 35 mL of N,N-dimethylformamide, heated to 145° C. with stirring, and TLC detected the reaction completion after 0.5 h. Filtration, concentration, washing with water, drying, dissolving in dichloromethane, and reprecipitation in petroleum ether to obtain 5.07 g of pure product with a yield of 83%.
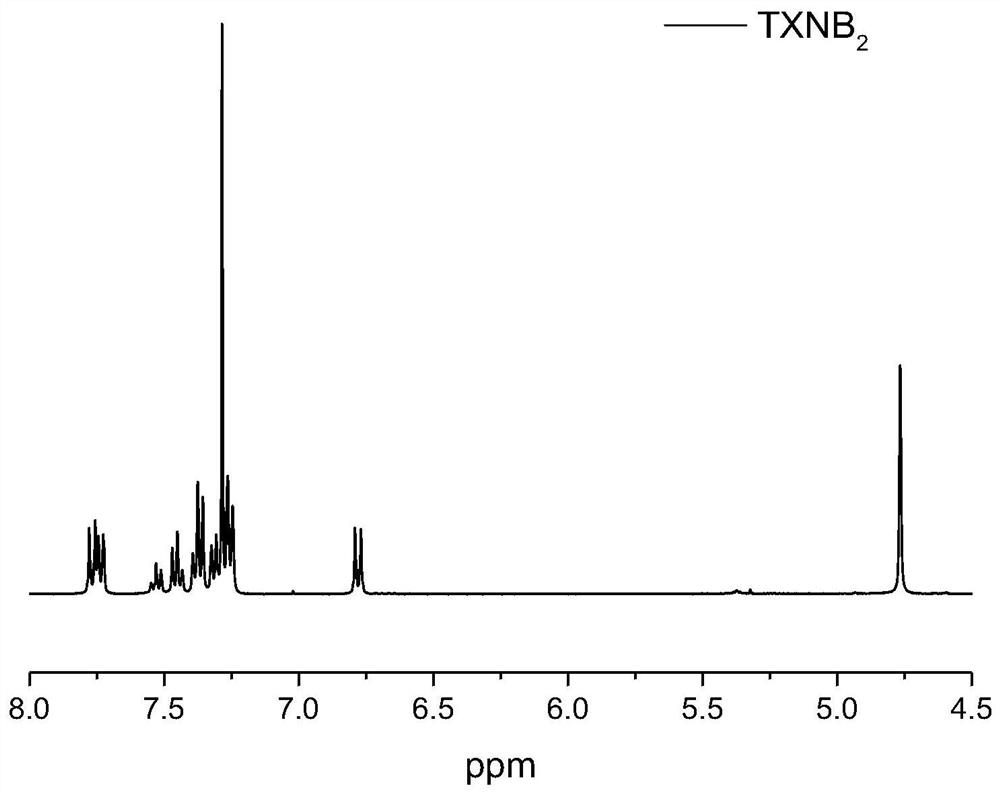
[0029] 1 H NMR (CDCl 3 , 400MHz) δppm (such as figure 1shown): 4.76(s, 4H), 6.75-6.80(d, J=8.80Hz, 2H), 7.22-7.27(m, 4H), 7.30-7.34(m, 2H), 7.34-7.40(m, 4H) ), 7.42-7.48(m, 2H), 7.50-7.56(t, J=7.2Hz, 1H), 7.71-7.79(m, 4H).
Embodiment 2
[0031] Preparation of N,N-bis(benzyl)thioxanthone photoinitiators
[0032] 2-Aminothioxanthone (3.41g, 15.0mmol), benzyl chloride (4.08g, 32.25mmol), pyridine (3.19g, 40.31 mmol), 68 mL of toluene, heated to 100° C. with stirring, and the completion of the reaction was detected by TLC after 12 h. Filtration, concentration, washing with water, drying, dissolving in dichloromethane, and reprecipitation in petroleum ether to obtain 5.61 g of pure product with a yield of 92%. The obtained pure product was confirmed to be N,N-bis(benzyl)thioxanthone from the data of H NMR spectrum.
Embodiment 3
[0034] Preparation of N,N-bis(benzyl)thioxanthone photoinitiators
[0035] 2-Aminothioxanthone (3.41g, 15.0mmol), benzyl chloride (4.18g, 33.0mmol), sodium carbonate (4.37g) were added to a 250ml three-necked flask equipped with a reflux condenser, a thermometer and a stirring bar. , 41.25 mmol), 40 mL of xylene, heated to 120° C. with stirring, and TLC detected the completion of the reaction after 10 h. Filtration, concentration, washing with water, drying, dissolving in dichloromethane, and reprecipitation in petroleum ether to obtain 5.68 g of pure product with a yield of 93%. The obtained pure product was confirmed to be N,N-bis(benzyl)thioxanthone from the data of H NMR spectrum.
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com