Flunarizine hydrochloride capsule preparation and preparation method thereof
A technology for flunarizine hydrochloride and preparation, which is applied in the field of flunarizine hydrochloride capsule preparation and preparation thereof, can solve the problem of unrealized, unsatisfactory requirements for consistency evaluation of generic drugs, unclear similarity and in vivo bioequivalence It can save manpower and material resources, have excellent stability, and achieve the effect of simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
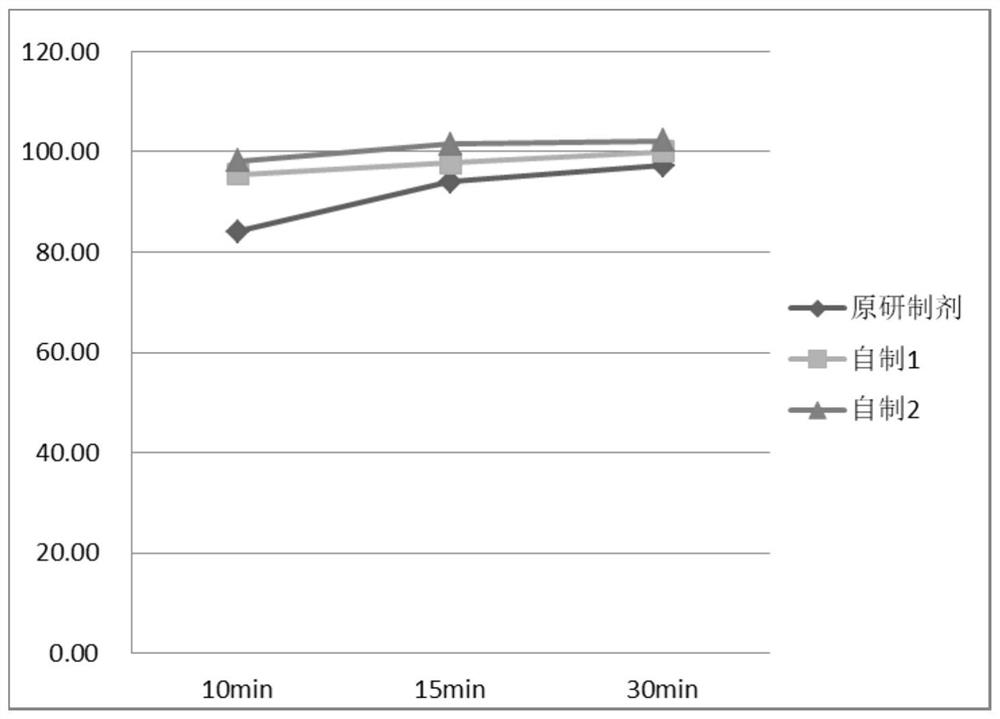
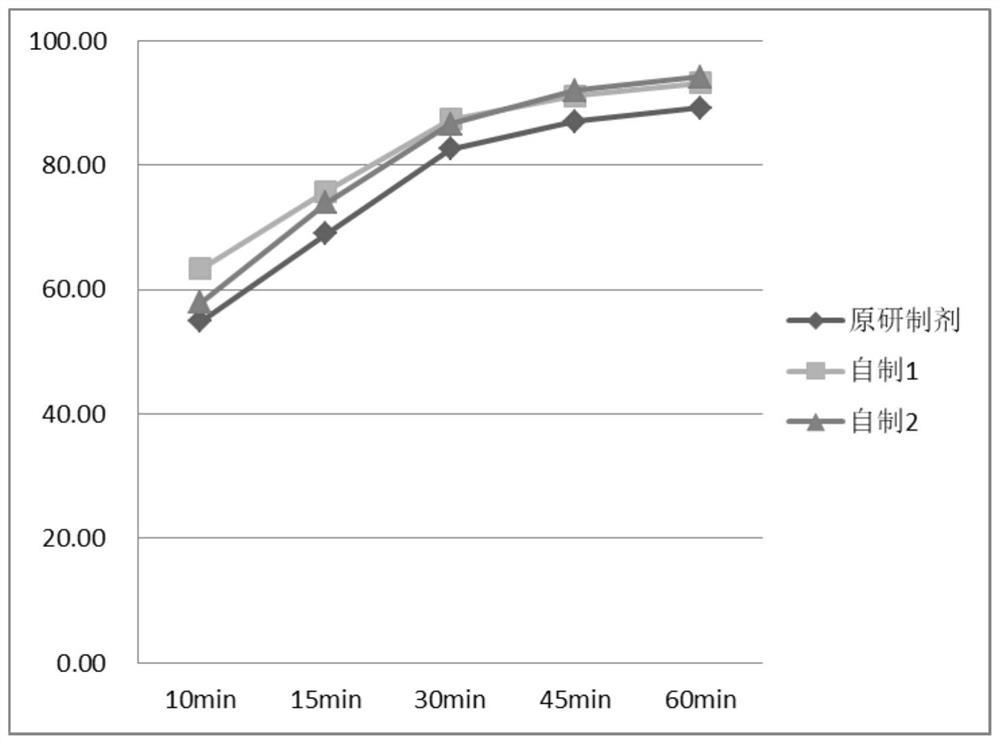
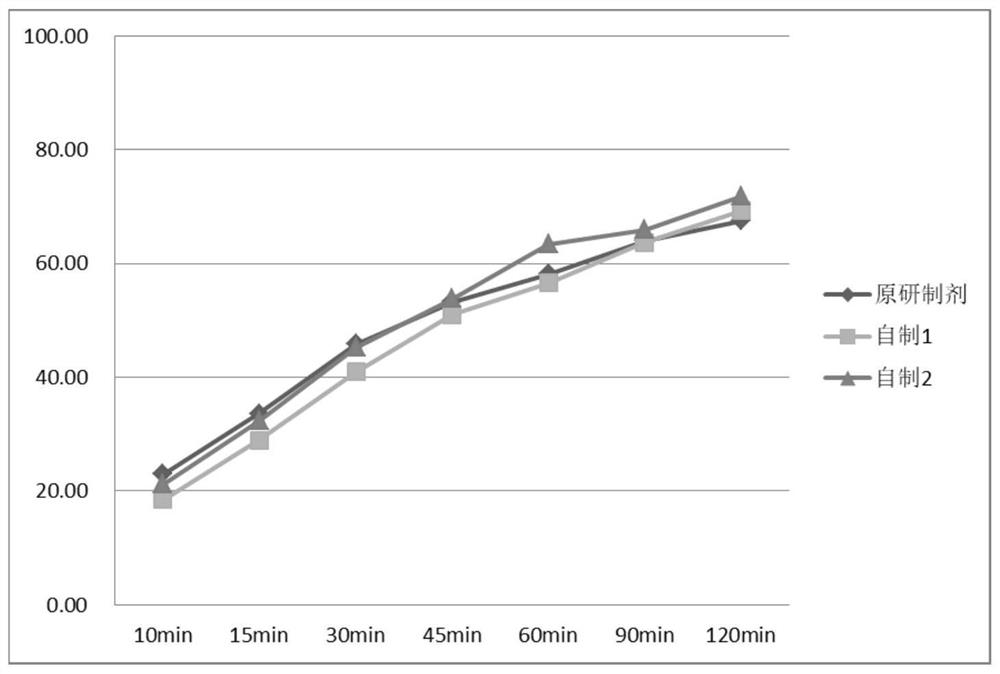
Image
Examples
Embodiment 1
[0038] 1. Quality composition of flunarizine hydrochloride preparation: 5.9 parts of flunarizine hydrochloride, 0.8 parts of silicon dioxide, 30 parts of corn starch, 15 parts of talcum powder, 18 parts of lactose GranuLac70, 90 parts of lactose GranuLac200, 3 parts of stearic acid magnesium.
[0039] 2. Preparation: Premix 0.59 kg of flunarizine hydrochloride and 0.08 kg of silicon dioxide for 10 minutes according to the formula, sieve through a granulator with a sieve aperture of 1.5 mm, then continue to mix for 15 minutes, and then add in equal increments 3kg of cornstarch, 1.8kg of lactose GranuLac70 and 9.0kg of lactose GranuLac200 were mixed evenly, and finally 1.5kg of talcum powder and 0.3kg of magnesium stearate were added, mixed evenly, and filled into capsules.
Embodiment 2
[0041] 1. Formula: 5.9 parts of flunarizine hydrochloride, 0.6 parts of colloidal silicon dioxide, 25 parts of corn starch, 12 parts of talcum powder, 29 parts of lactose GranuLac70, 87 parts of lactose GranuLac200, 1.0 part of magnesium stearate.
[0042] 2. Preparation: Premix 0.59kg of flunarizine hydrochloride, 0.06kg of colloidal silicon dioxide, 2.5kg of cornstarch and 1.2kg of talcum powder for 15 minutes, sieve through a granulator with a sieve aperture of 0.6mm, and continue mixing for 30 minutes , and then add 2.9kg of lactose GranuLac70 and 8.7kg of lactose GranuLac200 according to the method of equal increase, mix well, finally add 0.1kg of magnesium stearate, mix well and then fill the capsule.
Embodiment 3
[0044] 1. Formula: 5.9 parts of flunarizine hydrochloride, 0.5 parts of colloidal silicon dioxide, 20 parts of corn starch, 12 parts of talcum powder, 40 parts of lactose GranuLac70, 80 parts of lactose GranuLac200, 1.5 parts of magnesium stearate.
[0045] 2. Preparation: Premix 0.59kg of flunarizine hydrochloride, 0.05kg of colloidal silicon dioxide, and 2.0kg of corn starch according to the formula for 10 minutes, sieve through a granulator with a sieve aperture of 1.0mm, and then continue to mix for 20 minutes. Then add 4.0 kg of lactose GranuLac70 and 8.0 kg of lactose GranuLac200 in equal increments, mix well, and finally add 1.2 kg of talcum powder and 0.15 kg of magnesium stearate, mix well, and fill the capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com