Transdermal therapeutic system of huperzine A and derivatives thereof
A technology of huperzine A and a treatment system, applied in the field of treatment systems, can solve the problems of limiting the clinical application of HA and the inability of patients to take continuous medication, and achieves the effect of avoiding first-pass effect and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
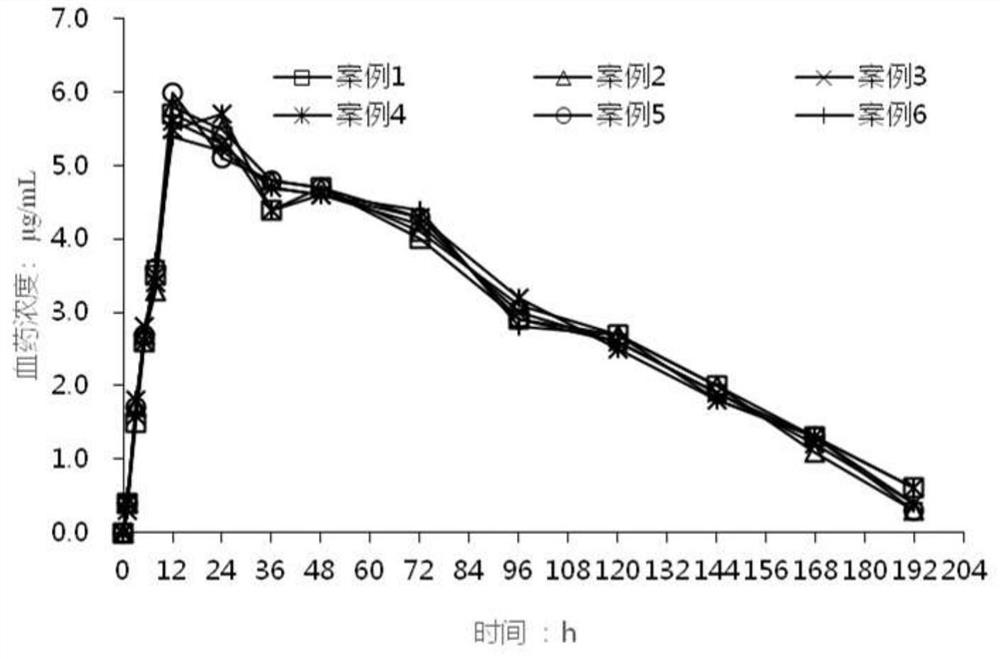
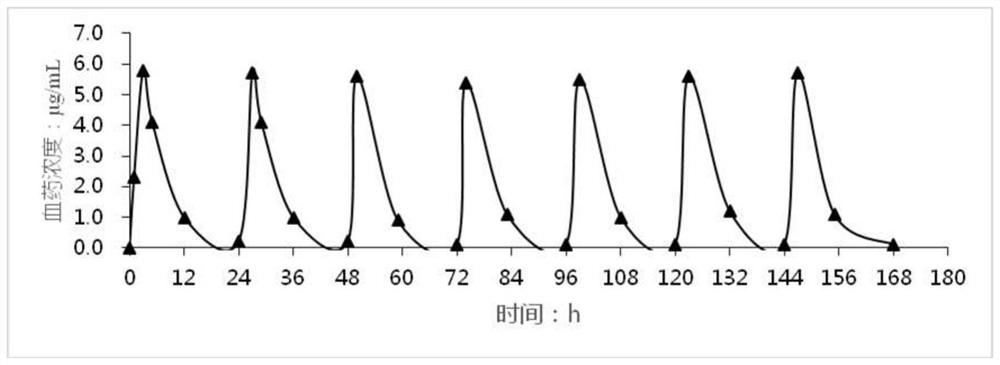
Image
Examples
Embodiment 1
[0026] The huperzine A transdermal patch for treating senile dementia of the present invention is prepared by the following prescription:
[0027]
[0028] Preparation process: Mix acrylate-vinyl acetate copolymer, ethyl acetate and huperzine A evenly, and use it for the coating of the active layer. A homogeneous coating of the active layer was applied on the siliconized membrane ("intermediate liner"), dried at 70° C. / 20 minutes, and covered with polyvinyl chloride. Weigh the silicone pressure sensitive adhesive adhesive and add the ethyl acetate, titanium dioxide, polyvinylpyrrolidone, and glycerin with stirring and stir until homogeneous for coating of the adhesive layer. A homogeneous coat of the adhesive layer was applied to the release layer and dried at 85°C / 5 minutes. The dried adhesive matrix is laminated to the medicated adhesive matrix of the active layer while removing the intermediate liner to prepare the final laminate. Transdermal patches of appropriate s...
Embodiment 2
[0030] The composition of the prescription is as follows:
[0031]
[0032] Preparation process: uniformly mix polypropylene ester, ethanol and huperzine A salt, and use it for the coating of the active layer. A homogeneous coating of the active layer was applied to the siliconized membrane ("intermediate liner"), dried at 70°C / 20 minutes, and covered with polyethylene. Weigh the silicone pressure sensitive adhesive adhesive and add the ethanol, silicon dioxide, polyvinylpyrrolidone and neutral oil with stirring and stir until homogeneous for coating of the adhesive layer. A homogeneous coat of the adhesive layer was applied to the release layer and dried at 85°C / 5 minutes. The dried adhesive matrix is laminated to the medicated adhesive matrix of the active layer while removing the intermediate liner to prepare the final laminate. Transdermal patches of appropriate size are punched out from the final laminate obtained.
Embodiment 3
[0034] The composition of the prescription is as follows:
[0035]
[0036]
[0037] Preparation process: uniformly mix polyisobutene, ethanol and huperzine A hydrate, and use it for the coating of the active layer. A homogenous coating of the active layer was coated on the siliconized membrane ("intermediate liner"), dried at 70°C / 20 minutes, and covered with polypropylene. Weigh the silicone pressure sensitive adhesive adhesive and add the ethanol, silicon dioxide, polyvinylpyrrolidone and neutral oil with stirring and stir until homogeneous for coating of the adhesive layer. A homogeneous coat of the adhesive layer was applied to the release layer and dried at 85°C / 5 minutes. The dried adhesive matrix is laminated to the medicated adhesive matrix of the active layer while removing the intermediate liner to prepare the final laminate. Transdermal patches of appropriate size are punched out from the final laminate obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com