Substituted polyacetylene grafted divinyl benzene microspherechiral chromatographic packing and preparation method thereof
A technology of divinylbenzene and chiral chromatography, which is applied in the field of chiral chromatography packing and its preparation, can solve problems such as poor stability, and achieve the effect of improving stability and avoiding damage to the helical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
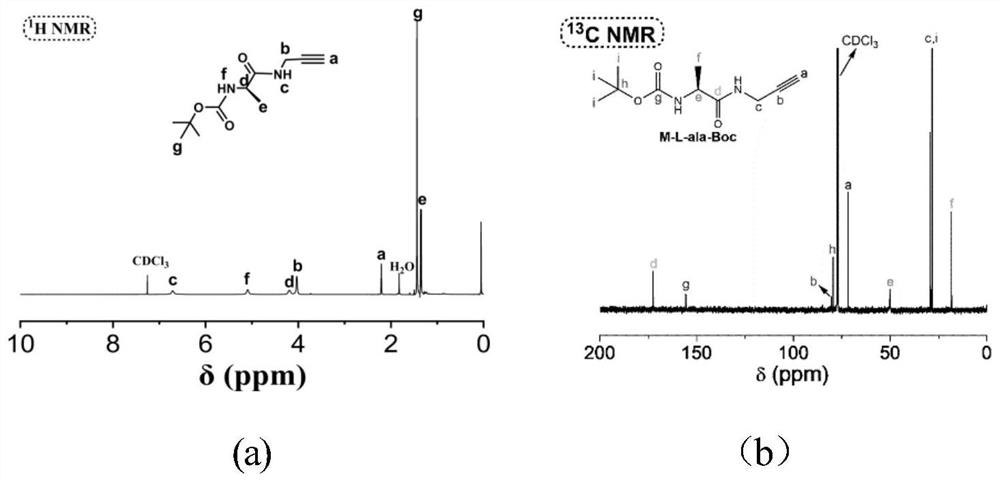
[0057] The first step to prepare M1 is as follows: Dissolve 1.90 g of N-Boc-alanine ((N-tert-butoxycarbonyl-alanine; CAS: 15761-38-3) in 40 mL of THF, then add 1.3 mL After reacting isobutyl chloroformate and 1.1mL N-methylmorpholine at 30°C for 15min, add 0.7mL propargylamine to the flask, react in a water bath at 30°C for 4h, and remove the formed precipitate by filtration; the filtrate was washed with ethyl acetate Ester was extracted, and washed three times with 30mL hydrochloric acid solution (2M), washed twice with 30mL saturated sodium bicarbonate solution, and once with 80mL deionized water, then dried with anhydrous magnesium sulfate, after vacuum distillation, in THF / n-hexane Purification by cooling and crystallization in a system with a volume ratio of 1 / 6. After 5 hours, filter and dry at 30° C. for 24 hours to 48 hours to obtain M1 as a solid product with a yield of 80%.
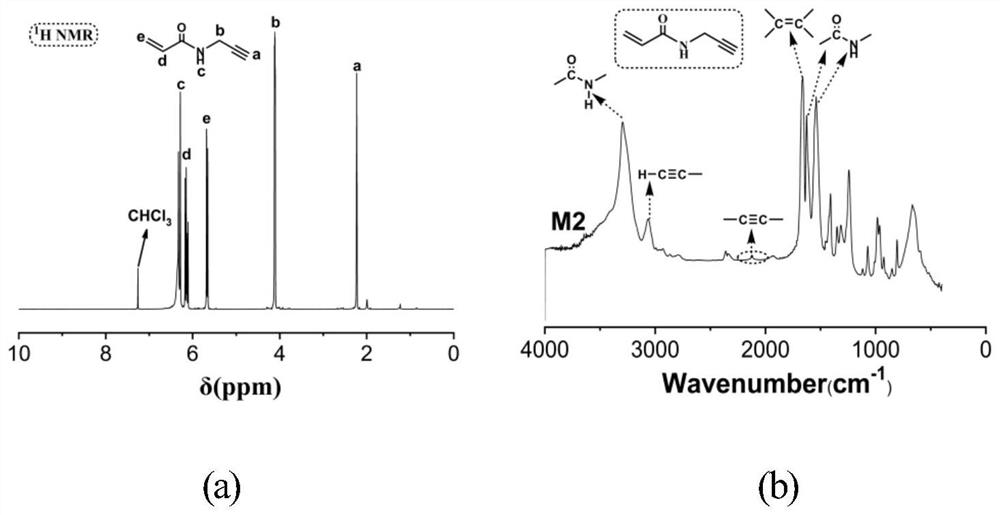
[0058] The second step to prepare M2 is as follows: dissolve 1.4ml of propargylamine and 4.2...
Embodiment 2
[0075] The first step is to prepare the substituted alkyne monomer M1, the specific method is as in Example 1.
[0076] The second step is to prepare the substituted alkyne monomer M2, the specific method is as example 1.
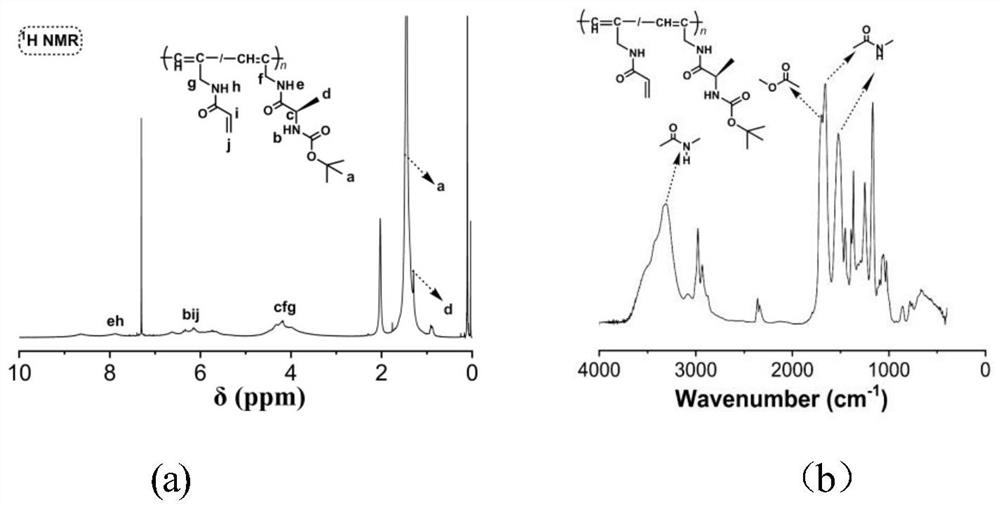
[0077] The third step is to prepare the substituted polyalkyne P-M1-co-M2-Boc, as follows: add 0.113g of M1, 0.055g of M2, 0.005g (nbd) Rh + B - (C 6 h 5 ) 4 Catalyst, and then the test tube was repeatedly evacuated and argon treated, a total of three times. Then add 5ml THF under the protection of argon, sonicate the test tube until the solid dissolves, then heat the test tube in a 30°C water bath, obtain a light red solution after polymerization for 6 hours, add the obtained solution dropwise to n-hexane to precipitate, filter After drying, precipitate twice in a THF / n-hexane volume ratio system of 1 / 6, filter, and dry to obtain yellow P-M1-co-M2-Boc with a yield of 76%.
[0078] The fourth step is to prepare the substituted polyalkyne P-M1-co-M2-NH...
Embodiment 3
[0084] The first step is to prepare the substituted alkyne monomer M1, the specific method is as in example 1.
[0085] The second step is to prepare the substituted alkyne monomer M2, the specific method is as example 1.
[0086] The third step is to prepare the substituted polyalkyne P-M1-co-M2-Boc as follows: add 0.136 g of M1, 0.044 g of M2, 0.005 g (nbd) Rh + B - (C 6 h 5 ) 4 Catalyst, and then the test tube was repeatedly evacuated and argon treated, a total of three times. Then add 5ml THF under the protection of argon, sonicate the test tube until the solid dissolves, then heat the test tube in a 30°C water bath, obtain a light red solution after polymerization for 6 hours, add the obtained solution dropwise to n-hexane to precipitate, filter After drying, precipitate twice in a THF / n-hexane volume ratio system of 1 / 6, filter, and dry to obtain yellow P-M1-co-M2-Boc with a yield of 78.2%.
[0087] The fourth step is to prepare the substituted polyalkyne P-M1-co-M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com