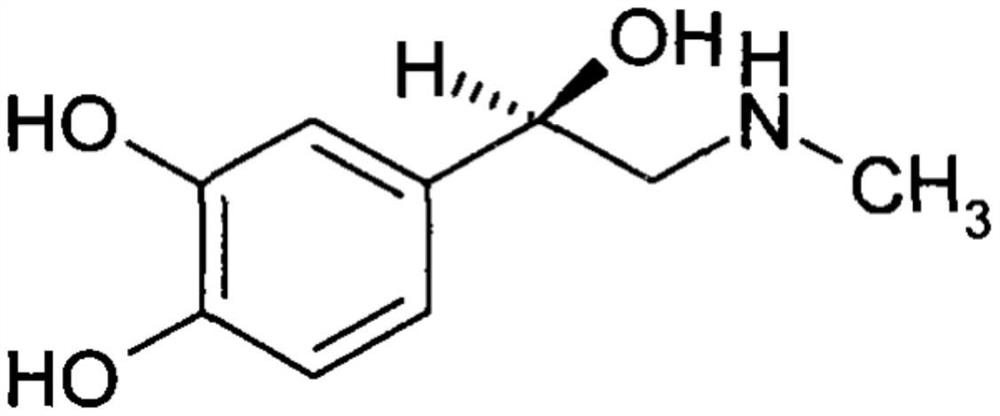
Adrenaline hydrochloride injection and preparation method thereof
A technology of epinephrine hydrochloride and epinephrine, which is applied in the field of medicine and can solve problems such as high cost, serious product quality problems, and harsh conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
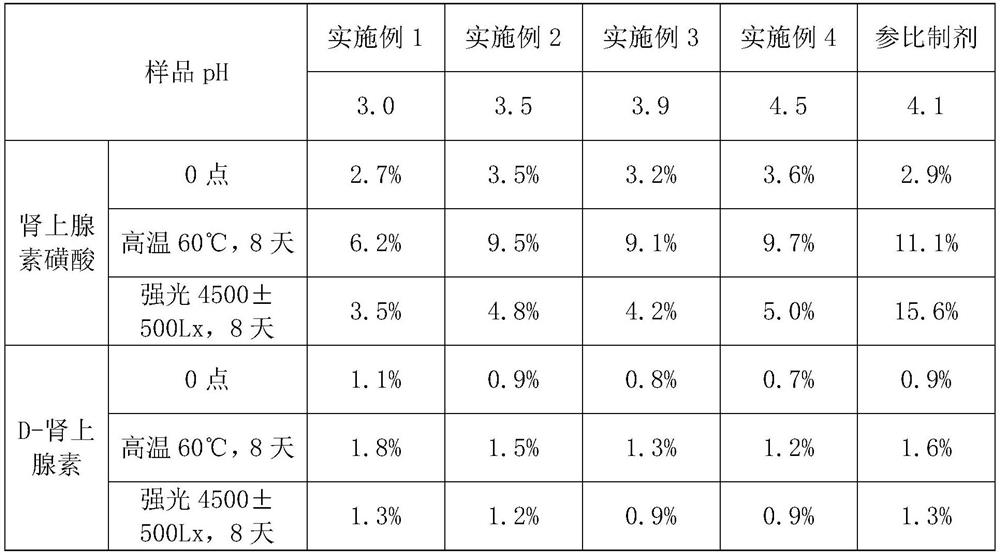
[0034] Components and proportioning ratio of embodiment 1-4 are shown in table 1:
[0035] Table 1 embodiment 1-4 component and proportioning
[0036] components Example 1 Example 2 Example 3 Example 4 adrenaline 1mg / ml 1mg / ml 1mg / ml 1mg / ml Sodium chloride 6.5mg / ml 6.0mg / ml 7.3mg / ml 8mg / ml Sodium metabisulfite 0.6mg / ml 0.8mg / ml 0.457mg / ml 0.2mg / ml L(+)-Tartrate 4.0mg / ml 3.5mg / ml 2.25mg / ml 0.1mg / ml Edetate disodium 0.4mg / ml 0.01mg / ml 0.2mg / ml 0.3mg / ml sodium hydroxide 2.1mg / ml 1.5mg / ml 1mg / ml 0.1mg / ml Hydrochloric acid solution qs to pH 3.0 qs to pH 3.5 qs to pH 3.9 qs to pH 4.5 Water for Injection Appropriate amount to 1ml Appropriate amount to 1ml Appropriate amount to 1ml Appropriate amount to 1ml
Embodiment 1
[0039] (1) Add 95% water for injection of the total amount in the preparation container, feed nitrogen to make the oxygen content less than or equal to 2ppm: vacuumize and discharge the air in the configuration container and pipeline, then feed nitrogen, after 3 cycles, Add water for injection to 95% of the total volume, pass nitrogen until the oxygen content in water for injection is less than or equal to 2ppm;
[0040] (2) Sodium hydroxide is dissolved with an appropriate amount of water for injection, and set aside;
[0041] (3) adding sodium chloride, sodium metabisulfite, L-(+) tartaric acid, disodium edetate, and the sodium hydroxide solution obtained in the above step (2) to the water for injection prepared in the above step (1);
[0042] (4) Add hydrochloric acid to adjust the pH of the solution obtained in step (3) to be 3.0, add epinephrine and stir to dissolve, add hydrochloric acid to keep the pH of the solution at 3.0, add water for injection to the total amount, ...
Embodiment 2
[0046] (1) Add 90% water for injection of the total amount in the preparation container;
[0047] (2) Sodium hydroxide is dissolved with an appropriate amount of water for injection, and set aside;
[0048] (3) Add sodium chloride, sodium pyrosulfite, L-(+) tartaric acid, disodium edetate, and the sodium hydroxide solution obtained in the above step (2) to the water for injection in the preparation container in the above step (1) ;
[0049] (4) Add hydrochloric acid to adjust the pH of the solution obtained in step (3) to be 3.5, add epinephrine and stir to dissolve, add hydrochloric acid to keep the pH of the solution at 3.5, add water for injection to the total amount, and feed nitrogen to control the oxygen content of the injection to be less than or equal to 2ppm ;
[0050] (5) The injection obtained in step (4) is filtered through a 0.45 μm microporous membrane once, and then twice through a 0.22 μm microporous membrane;
[0051] (6) The injection liquid obtained in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com