Novel thiazole azo dye and preparation method thereof
A technology for thiazole azo and dyes, applied in the direction of azo dyes, monoazo dyes, organic dyes, etc., to achieve the effects of increasing yield, improving stability, improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
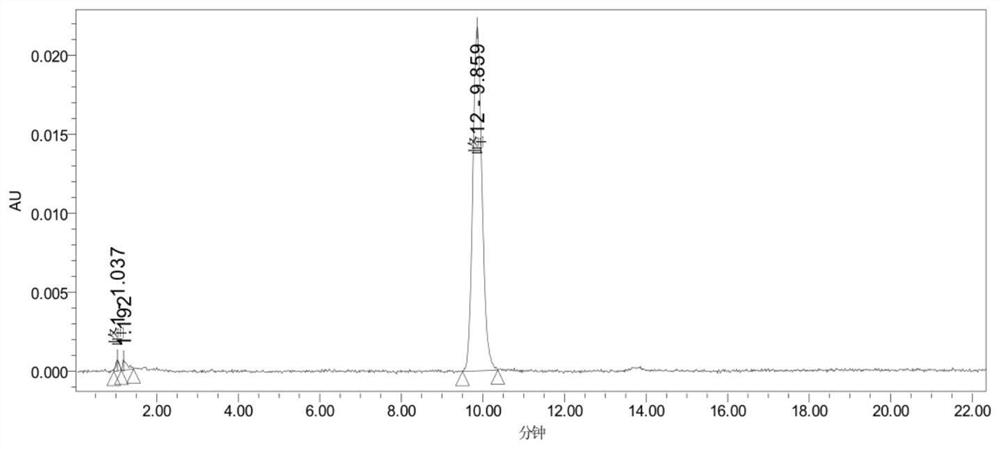
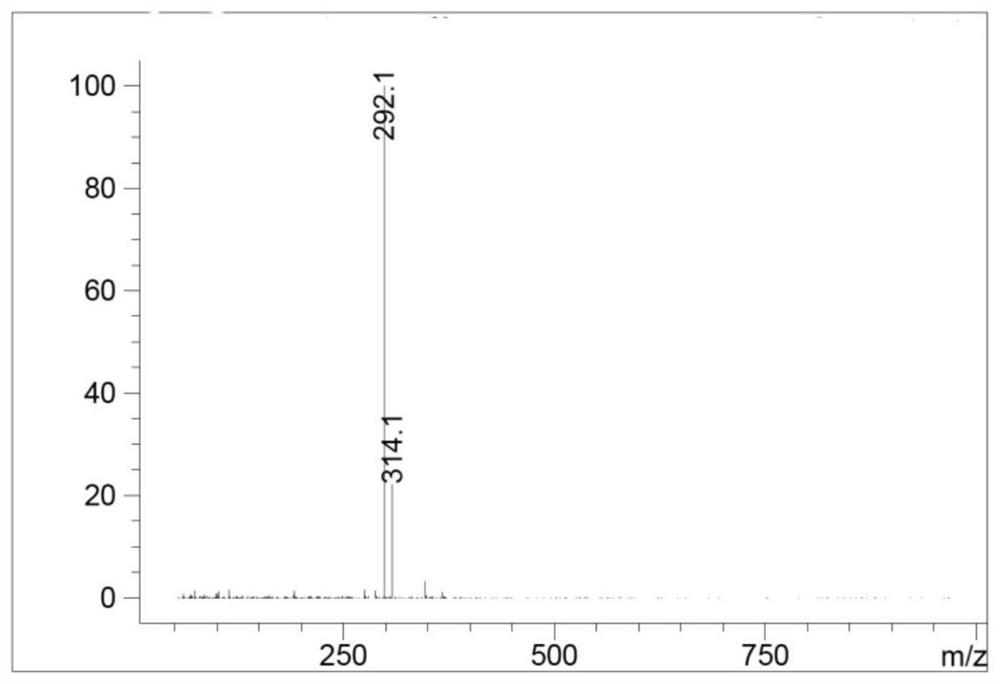
Image
Examples
Embodiment 1
[0041] Examples 1-16 provide a novel thiazole azo dye represented by the formula (1), and the following uses Example 1 as an example for illustration.
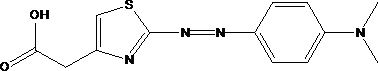
[0042] The novel thiazole azo dyes shown in formula (1) provided by embodiment 1 have a structural formula as shown in formula (1):
[0043]
[0044] The preparation steps of the thiazole azo dyes shown in the formula (1) are:
[0045] Get 15.8g (0.1mol) (2-aminothiazol-4-yl) acetic acid, dissolve with the concentrated phosphoric acid of 80wt% mass fraction with 252.8g, after completely dissolving, cool to 0 ℃, add 6.9g sodium nitrite ( 0.1mol), and then reacted at 0°C for 2h to obtain a diazotization reaction solution; 12.1g N,N-dimethylaniline (0.1mol) was dissolved in 100mL methanol solution, cooled to 0°C to obtain the cooled The methanol solution of N,N-dimethylaniline; add the cooled methanol solution of N,N-dimethylaniline into the diazotization reaction liquid, continue the reaction at 0°C for 2 hours, and pour 500...
Embodiment 2
[0047] Example 2 is different from Example 1 only in that: the temperature is replaced by -5°C from 0°C.
Embodiment 3
[0048] Embodiment 3, differs from Embodiment 2 only in that: the mass fraction of the concentrated phosphoric acid is 85wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com