Betamethasone sodium phosphate injection and preparation method and application thereof
A technology of sodium metasone phosphate and injection, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as safety issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] In a second aspect, embodiments of the present invention provide a method for preparing betamethasone sodium phosphate injection according to the first aspect, comprising the following steps:
[0037] S1: Betamethasone sodium phosphate, edetate disodium, disodium hydrogen phosphate and 1% phosphoric acid solution are prepared in formula quantities;
[0038] S2: Add water for injection with a formula amount of 60% to 90% into the liquid preparation tank, fill with nitrogen, then add and dissolve the edetate disodium, the betamethasone sodium phosphate, the disodium hydrogen phosphate and The 1% phosphoric acid solution is supplemented with the water for injection to the prescribed amount to obtain a medicinal solution;
[0039] S3: Pass the medicinal solution through a sterilizing filter and put it into a vial or ampoule purged with nitrogen to obtain an injection with a residual oxygen content in the headspace ≤ 4%, and the pH of the injection is 8.2- 8.5.
[0040] Th...
Embodiment 1~4
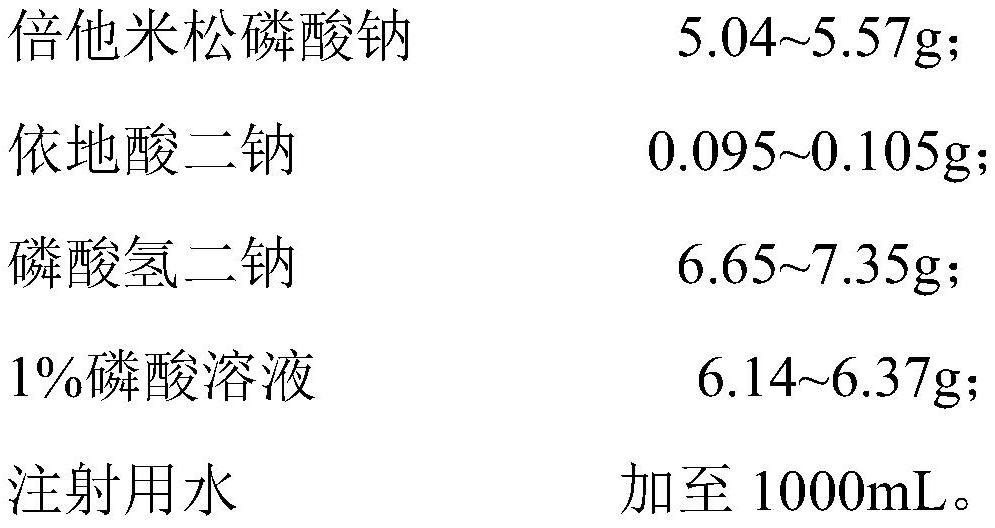
[0061] Based on 1000 mL of betamethasone sodium phosphate injection, the contents of the components of the betamethasone sodium phosphate injection provided in Examples 1-4 are shown in Table 1.
[0062] Table 1
[0063]
[0064] Betamethasone sodium phosphate injection was prepared respectively according to Preparation Method 2. It can be seen from Table 1 that when the dosage of disodium hydrogen phosphate is 6.65-7.35 mg / mL, the osmotic pressure is consistent with that of the reference preparation, and the dosage of disodium hydrogen phosphate is 6 mg / mL , the osmotic pressure differed greatly from that of the reference preparation.
Embodiment 5~8
[0066] Based on 1000 mL of betamethasone sodium phosphate injection, the contents of the components of the betamethasone sodium phosphate injection provided in Examples 5-8 are shown in Table 2.
[0067] Table 2
[0068]
[0069] Prepare betamethasone sodium phosphate injection respectively according to preparation method 8, respectively take the betamethasone sodium phosphate injection prepared in Examples 5 to 8, at a temperature of 40°C ± 2°C and a relative humidity of 75% ± 5%. Stand for 30 days, carry out accelerated test, measure impurity and main drug content in betamethasone sodium phosphate injection according to high performance liquid chromatography (Chinese Pharmacopoeia 2020 edition four general rules 0512), measurement result is shown in Table 3.
[0070] table 3
[0071]
[0072] It can be seen from Table 3 that when the dosage of edetate disodium is 0.095-0.105mg / mL, the growth rate of betamethasone and total impurities is small, and the stability of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dissolved oxygen | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com