Drying method of imidazolyl fluorine-containing lithium salt
A drying method, imidazole-based technology, applied in the drying field of imidazole-based fluorine-containing lithium salt, can solve the problems of moisture sensitivity, poor thermal stability and chemical stability, etc., achieve mild reaction conditions, shorten vacuum drying time, and avoid reaction side effects The effect of product formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
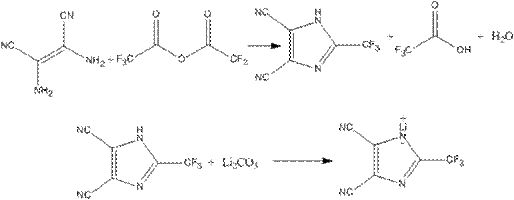
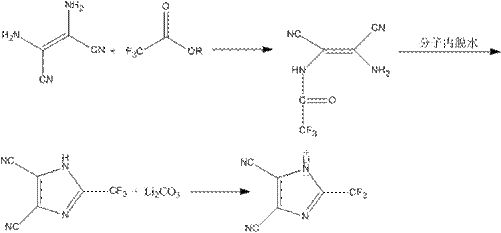
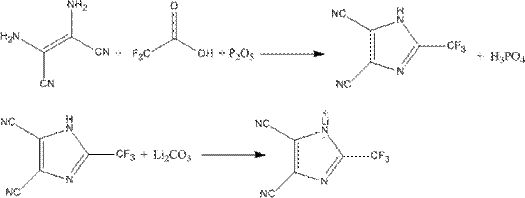
[0026] Step 1: In a 500ml four-necked flask, add 50g of diaminomaleonitrile and 250ml of anhydrous acetonitrile, then drop in 102g of trifluoroacetic anhydride, raise the temperature to 50°C and stir for 4 hours, then distill off the solvent and by-products under reduced pressure to obtain 77.4 g of 2-trifluoromethyl-4,5-dicyanoimidazole.
[0027] Step 2: In a 500ml four-necked flask, 16.8g of lithium carbonate was made into an aqueous phase suspension, and 77.4g of 2-trifluoromethyl-4,5-dicyanoimidazole prepared in step 1 was added, and the temperature was raised to 40°C. Continue to stir the reaction for 0.5h, the pH test paper test reaction solution is neutral, then add 5g of activated carbon for decolorization, raise the temperature to 70°C, continue to stir for 2h, filter while it is hot, cool to room temperature, and filter again to obtain 2-trifluoromethyl-4, 70.3 g of lithium salt of 5-dicyanoimidazole.
[0028] Step 3: Take 70.3g of 2-trifluoromethyl-4,5-dicyanoimida...
Embodiment 2
[0031] Step 1 and step 2 in Example 1 were repeated, and the recovered absolute ethanol was added in step 3. The obtained lithium salt of 2-trifluoromethyl-4,5-dicyanoimidazole was vacuum-dried at 135° C. for 3 hours, and the water content in the Karl Fischer test was 17 ppm.
Embodiment 3
[0032] Embodiment 3 (comparative example):
[0033] Repeat steps 1, 2, and 4 in Example 1, rinse with absolute ethanol, and dry the lithium salt of 2-trifluoromethyl-4,5-dicyanoimidazole in vacuum at 150°C for 8 hours. Hugh tested for a moisture content of 150ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com