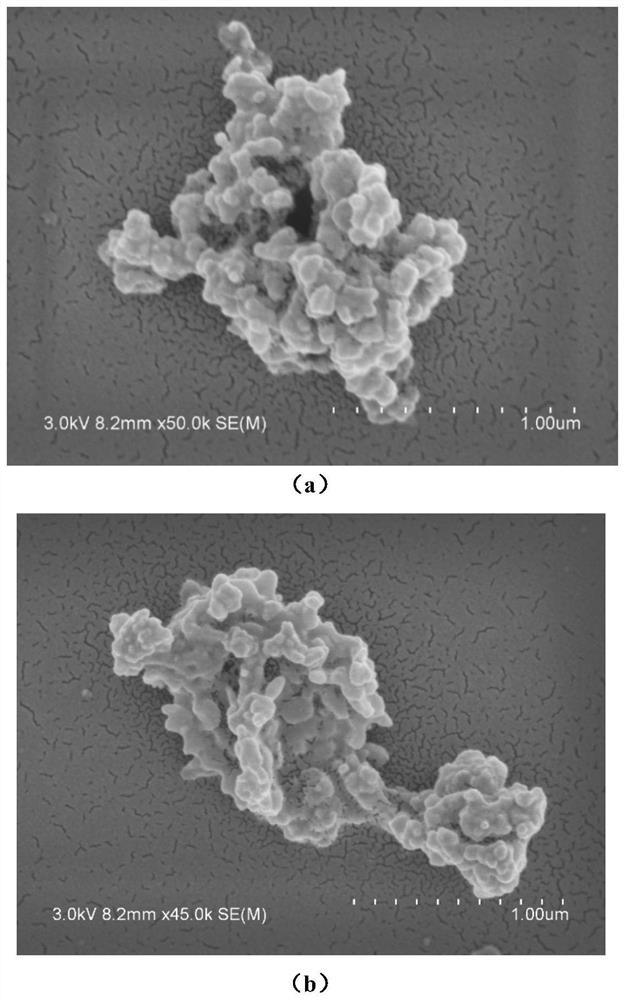
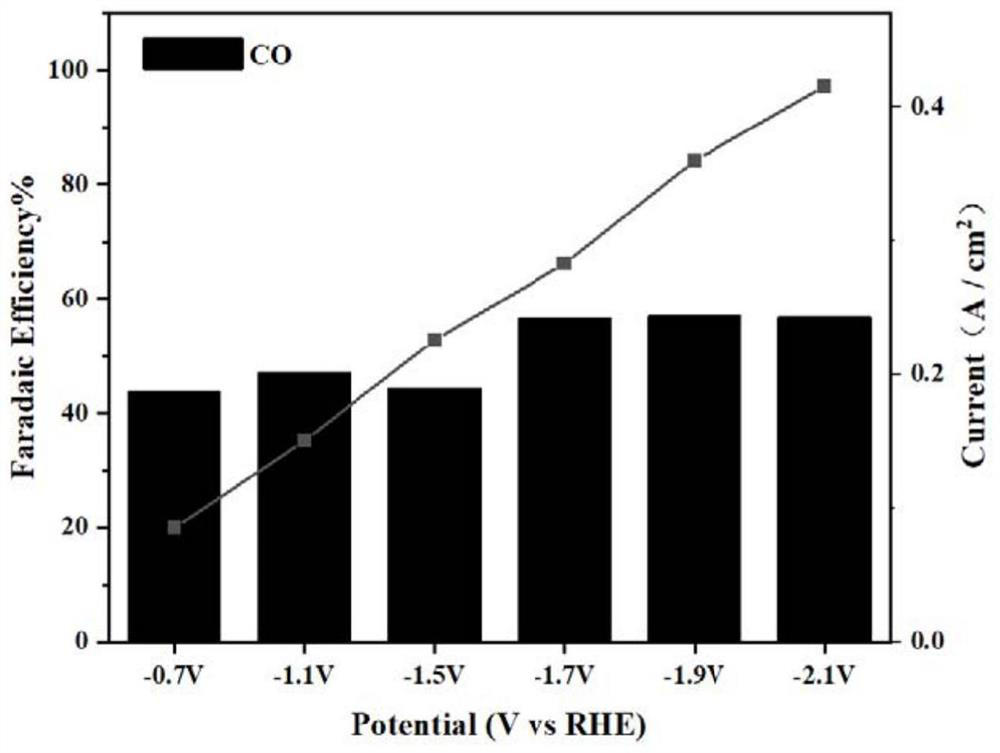
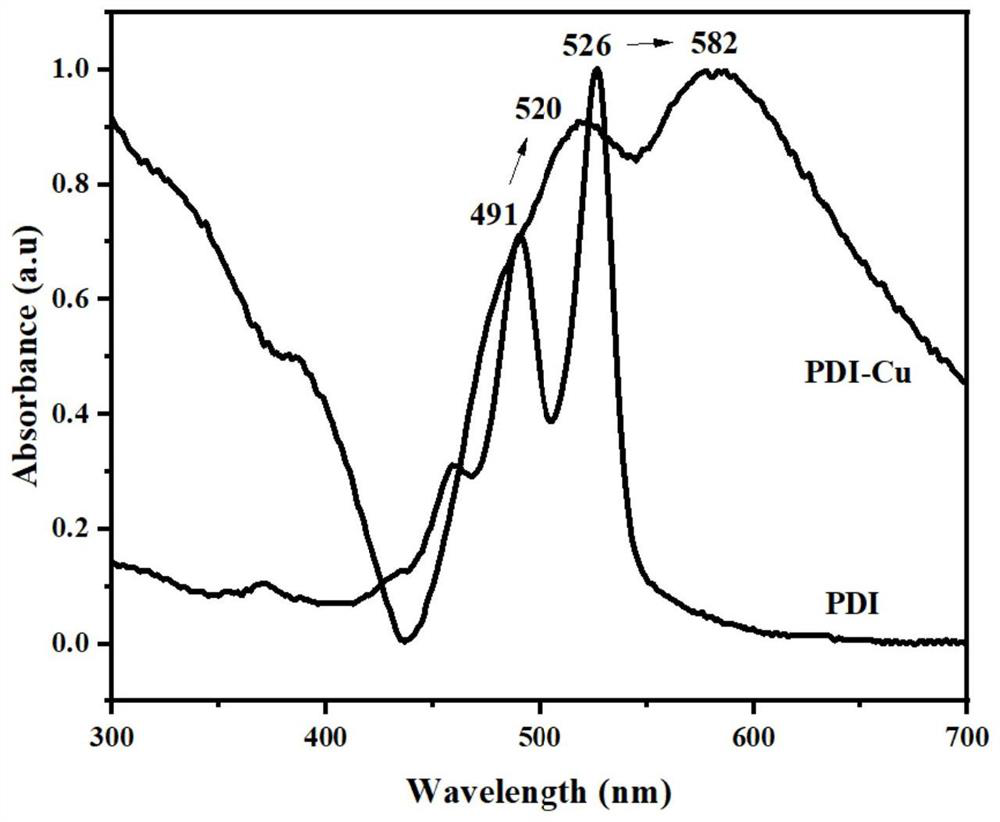
Cu-MOF material for electrocatalytic reduction of CO2 and preparation method of Cu-MOF material
An electrocatalysis, CO2 technology, applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve the problems of low yield, poor catalyst stability, low selectivity of catalytic products, etc., to achieve extended durability and good industrial application prospects. , the effect of improving the catalytic rate and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Take 5g of 3,4,9,10-perylenetetraic acid dianhydride, add it to 100mL N,N-dimethylformamide solution, stir and then add dropwise 15mL 1-(3-aminopropyl)imidazole , heated to 140°C, condensed and refluxed, stirred at a constant temperature in an inert gas environment for 12 hours, filtered and washed with tetrahydrofuran to remove 1-(3-aminopropyl)imidazole remaining on the surface, put it into a vacuum oven, raised to 60°C, and kept at a constant temperature Drying for 8 hours to remove residual THF gave the PDI product as a red powder.
[0028] (2) Get 4g of the PDI prepared in step (1), add 300mL of chloroform solution, seal and sonicate for 0.5h, obtain the chloroform supersaturated solution of PDI, filter with a sand core filter, remove the filter cake, collect The filtrate with fluorescent color is a chloroform saturated solution of PDI;
[0029] Dissolve an appropriate amount of copper chloride dihydrate in methanol to prepare a methanol solution of copper chl...
Embodiment 2
[0035](1) Take 3g of 3,4,9,10-perylenetetraic acid dianhydride, add it to 100mL N,N-dimethylformamide solution, stir and then add 10mL 1-(3-aminopropyl)imidazole dropwise, Heat up to 140°C, condense and reflux, stir at constant temperature for 12 hours in an inert gas environment, filter and wash with tetrahydrofuran to remove 1-(3-aminopropyl)imidazole remaining on the surface, put it in a vacuum oven, heat up to 60°C, and dry at constant temperature 8 hours to remove residual THF to give the PDI product as a red powder.
[0036] (2) Get 2g of the PDI prepared in step (1), add 150mL of chloroform solution, seal and sonicate for 0.5h, obtain the chloroform supersaturated solution of PDI, filter with a sand core filter, remove the filter cake, and collect The filtrate with fluorescent color is a chloroform saturated solution of PDI;
[0037] Dissolve an appropriate amount of copper chloride dihydrate in methanol to prepare a methanol solution of copper chloride dihydrate with ...
Embodiment 3
[0041] (1) Take 4g of 3,4,9,10-perylenetetraic acid dianhydride, add it to 50mL N,N-dimethylformamide solution, stir and then add dropwise 10mL 1-(3-aminopropyl)imidazole , heated to 120°C, condensed and refluxed, stirred at a constant temperature in an inert gas environment for 16 hours, filtered and washed with tetrahydrofuran to remove 1-(3-aminopropyl)imidazole remaining on the surface, put it into a vacuum oven, raised to 80°C, and kept at a constant temperature Drying for 4 hours to remove residual THF gave the PDI product as a red powder.
[0042] (2) Get 4g of the PDI prepared in step (1), add 300mL of chloroform solution, seal and sonicate for 1h, obtain the chloroform supersaturated solution of PDI, filter with a sand core filter, remove the filter cake, and collect The filtrate of fluorescent color is a chloroform saturated solution of PDI;
[0043] Dissolve an appropriate amount of copper chloride dihydrate in methanol to form a methanol solution of copper chlorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com