Sulfonic polymer eutectic solid electrolyte and preparation method thereof
A solid electrolyte and sulfonic acid-based technology, applied in solid electrolytes, non-aqueous electrolytes, non-aqueous electrolyte batteries, etc., can solve the problems of failure to meet practical application requirements, breakthrough improvement in electrolyte conductivity, high dissociation transition energy barrier, etc. problems, achieve the effect of realizing large-scale continuous production and equipment, expanding the scope of use, and improving ion transmission efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
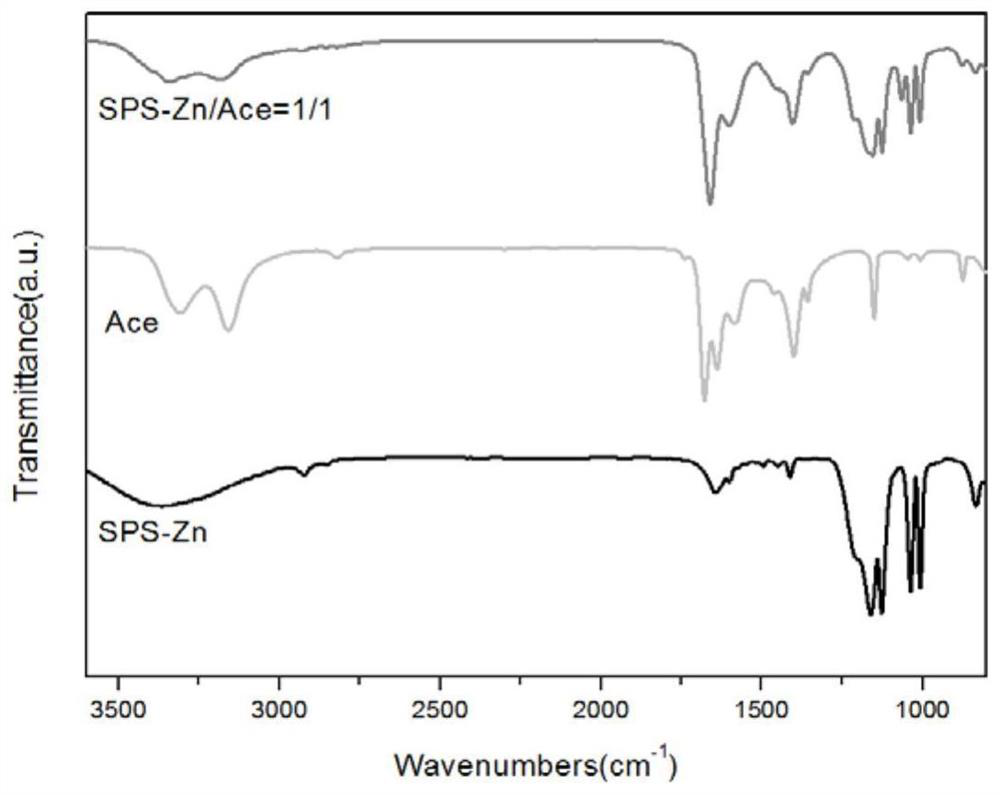
[0059] Polystyrene sulfonate and the acetamide at different mass ratings were mixed with acetamide, and different mass ratios were 1 / 0.8, 1 / 1, 1 / 1 / 1 / 2, and then placed under a high temperature environment (80 ° C) stir, After the mutual dissolution, cool to room temperature, resulting in a uniform mass of polystyrenesulfonate and acetamide polymer, melt electrolyte (see figure 2 ).
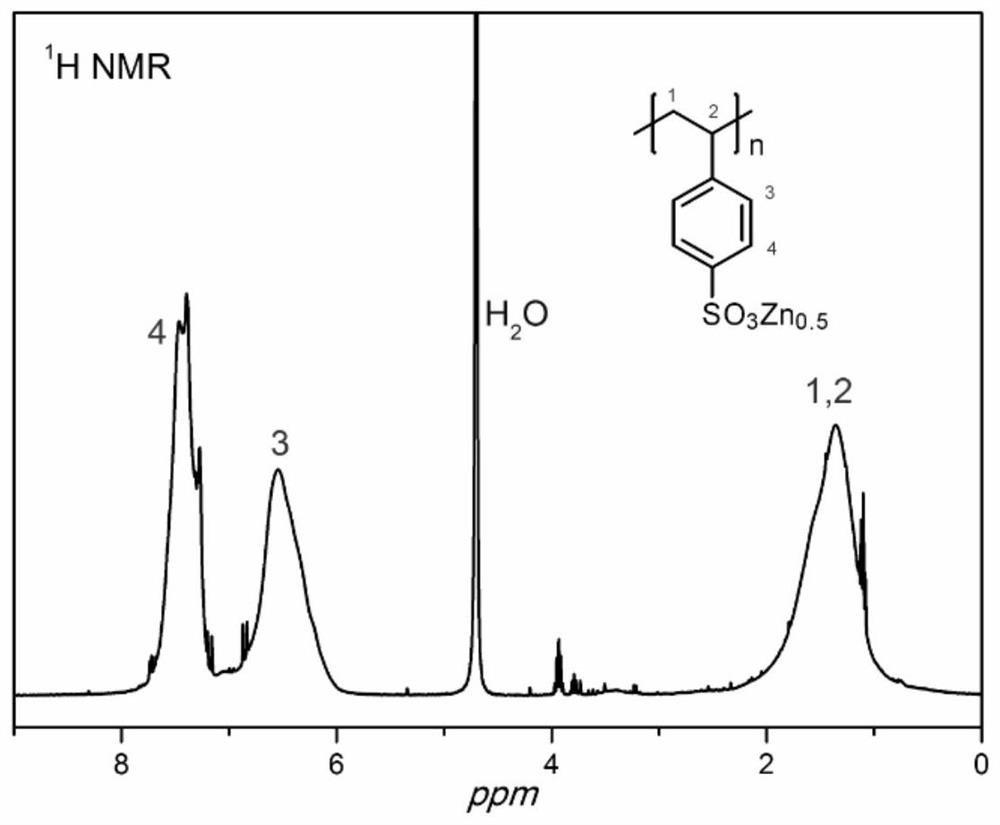
[0060] The polystyrenesulfonate preparation process: aqueous solution of polystyrene sulfonic acid is blended with zinc sheet, stirred for 1 day, filtered, and dried in vacuo. The structure of the product is characterized, that is, the zinc content in the sample is titrated, theoretical calculations and ICP measurements, as shown in Table 1.
[0061] Table 1 Zinc concentration test in zinc polystyrenesulfonate
[0062] Test Methods WT.% a
WT.% b
WT.% c
Zinc ion content 17.2 16.7 17.0
[0063] a Theoretical calculation b ICP analysis calculation c Zinc ion titration
...
Embodiment 2
[0069] 1.00g of polystyrenesulfonate, 1.00 g of acetamide and 0.05 g of TiO 2 The powder is mixed at room temperature, and then placed under a high temperature environment (80 ° C) stir, the mutually soluble union, cool to room temperature, to obtain zinc polystyrenesulfonate, acetamide, and TiO 2 Polymer coputical electrolyte.
Embodiment 3
[0071] 0.45 g of polystyrene sulfonate and 0.50 g of acetamide were mixed, and then stirred at a high temperature environment (80 ° C), the mutiny was mixed, cooled to room temperature to obtain polystyrene sulfonate and acetamide Polymer coputical electrolyte.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com