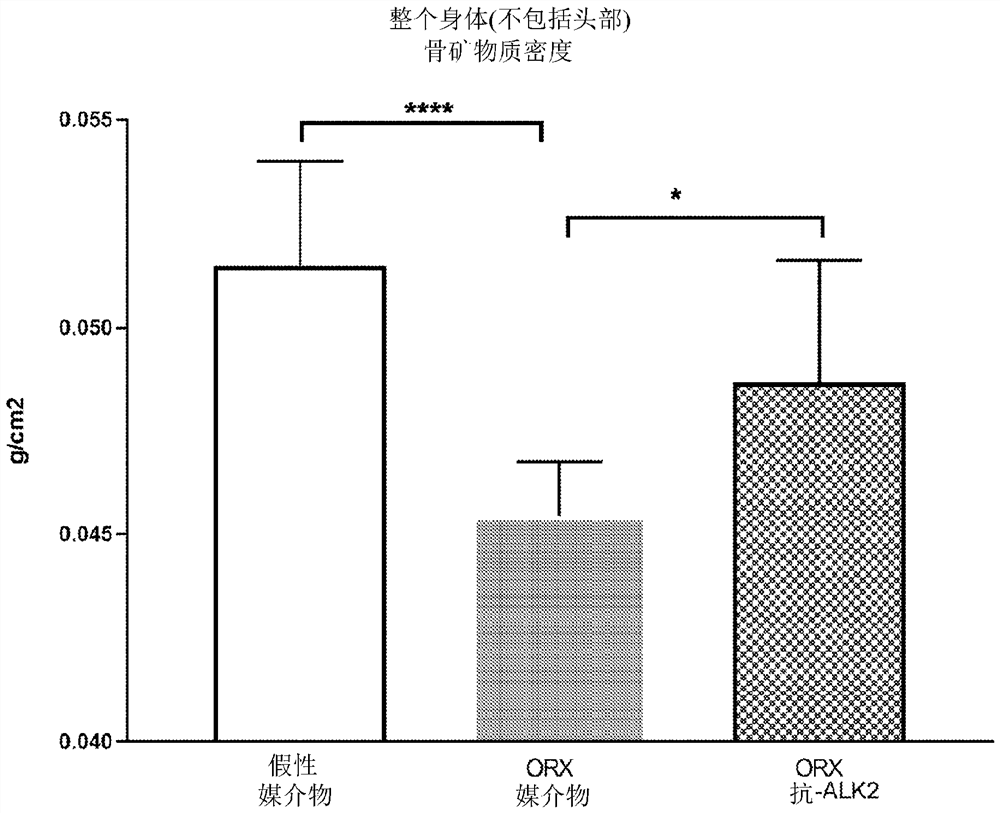
Alk2 antibodies and methods of use thereof
An antibody, CDR2 technology, applied in the direction of antibodies, chemical instruments and methods, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve regression, lack of efficacy, lack of effectiveness in reducing or reversing cardiac fibrosis therapy and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0525] Example 1 - Generation of ALK2 Antibodies
[0526] Antibodies were generated by panning a human combinatorial phage library against antibodies recognizing ALK2, followed by 2 rounds of enrichment and counter-selection against closely related antigens to deplete non-specific antibodies. After the second round of enrichment, rapid pool maturation "RAPMAT®" (Prassler et al., Immunotherapy, 1(4), 571-583, 2009) was performed (RAPMAT® is a registered trademark of MorphoSys AG). A highly diverse set of CDR variability is cloned into the selected antibody pool, thus further expanding the diversity of the preselected antibody pool. The amplified antibody library panned against ALK2 was enriched using high stringency wash conditions and 2 additional rounds to reduce the amount of antigen in the panning round, and counterselected against closely related antigens to deplete non-specific antibodies. After panning, the enriched antibody gene pool was subcloned from a phage display ...
Embodiment 2
[0528] Example 2 - Evaluation of ALK2 Antibody Binding Affinity by Surface Plasmon Resonance (SPR)
[0529] A GE Biacore 3000 was used to measure the kinetics of the interaction between anti-ALK2 antibody (ligand) and ALK2-His / ALK2-mFC (analyte). Flow cells 1-4 were immobilized with anti-human capture antibody from GE using an amine coupling kit. Anti-ALK2 protein was then captured on the chip in flow cells 2-4, with flow cell 1 left empty as a reference cell to measure and subtract any non-specific binding. When running kinetic analyzes on Biacore, it is best to allow a maximum analyte binding response (RMax) of 100 resonance units (RU). This is achieved by the formula RMax100=(kDa analyte / kDa ligand) x immobilized ligand RU. If the ligand density in the flow cell is too high, it will result in an artificially low concentration of unbound analyte close to the chip surface, and the resulting sensorgram will not represent the true binding between ligand and analyte. This is ...
Embodiment 3
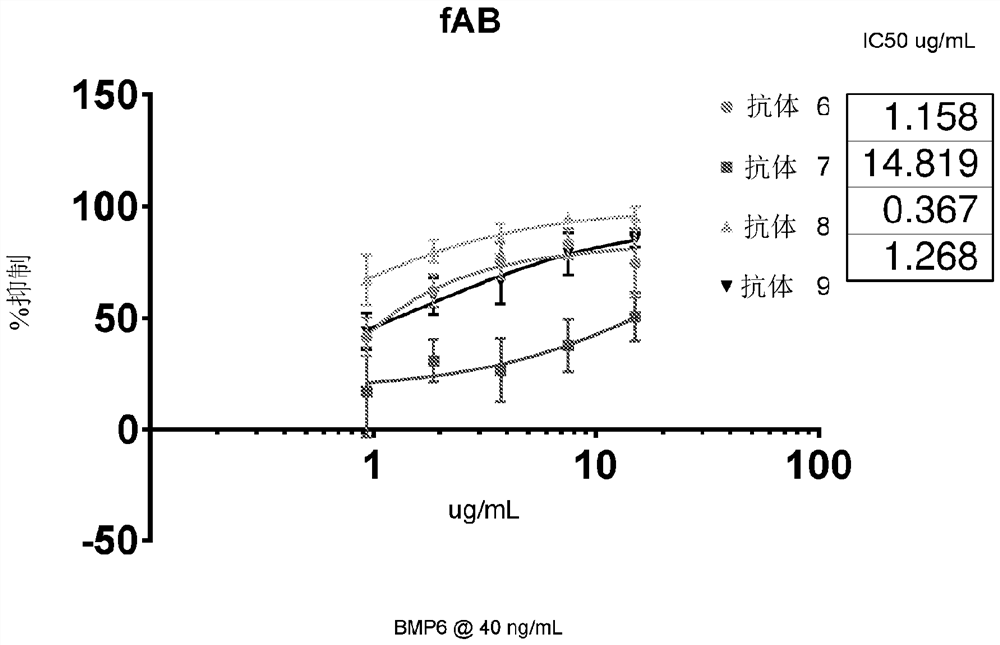
[0534] Example 3 - Evaluation of ALK2 Antibody Detection Using a Genetic Luciferase Reporter Assay
[0535] C2C12-BRE-luciferase (SMAD1 reporter gene) cells were seeded on 96-well plates in DMEM supplemented with 2% FBS, and placed in the incubator for no less than 3 hours to adapt to the plate surface. For each fAb variant, a dilution series was prepared in 2% DMEM and administered onto cells. The remaining wells were used for duplicate positive controls and background. Cells were then placed in a 37°C incubator for 45 minutes. Wells were treated with vehicle (to determine background), BMP6 (to determine ALK2 stimulation), or fAB followed by 40 ng / mL BMP6 (to determine IC50 of fAb). As a surrogate for SMAD1 phosphorylation, the extent of BRE-stimulated luciferase was measured. Plates were incubated overnight and then read using Promega Steady Glo and Molecular Devices Spectramax M5e. Inhibition was calculated as percent signal loss compared to positive control wells. Fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com