Pharmaceutical composition, sustained release preparation and preparation method thereof
A technology for sustained-release preparations and compositions, applied in the field of sustained-release preparations based on liquid crystal drug delivery systems and their preparation, and in the field of peptide pharmaceutical compositions, can solve the problems that the repeatability and uncontrollability of the production process cannot be guaranteed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of a pharmaceutical composition, which contains a complex (preferably covalently conjugated) of a pharmaceutically acceptable salt of a peptide drug active substance and one or more lipophilic or amphiphilic carrier macromolecular salts ( (hereinafter referred to as "Compound")
[0057] 1.1 Taking Octreotide Acetate (OCT), Leuprolide Acetate (LB), Cetrorelix Acetate (RK) and Liraglutide (LT, its acetate) as examples respectively, the preparation containing them respectively with The pharmaceutical composition of the complex formed by sodium dodecyl sulfate (SDS), palmitic acid (PA) or carboxymethylcellulose (CMC):
[0058] 1.1.1 Preparation of a pharmaceutical composition containing complex 1 (octreotide modified by sodium lauryl sulfate, SDS-OCT)
[0059] Prepare 100 mL of 0.1% sodium lauryl sulfate aqueous solution. Prepare 100 mL of 0.2% octreotide acetate aqueous solution. After fully mixing 100 mL of octreotide acetate solution with 100 mL...
Embodiment 2
[0122] Example 2: Preparation of depot compositions for liquid crystal drug delivery systems
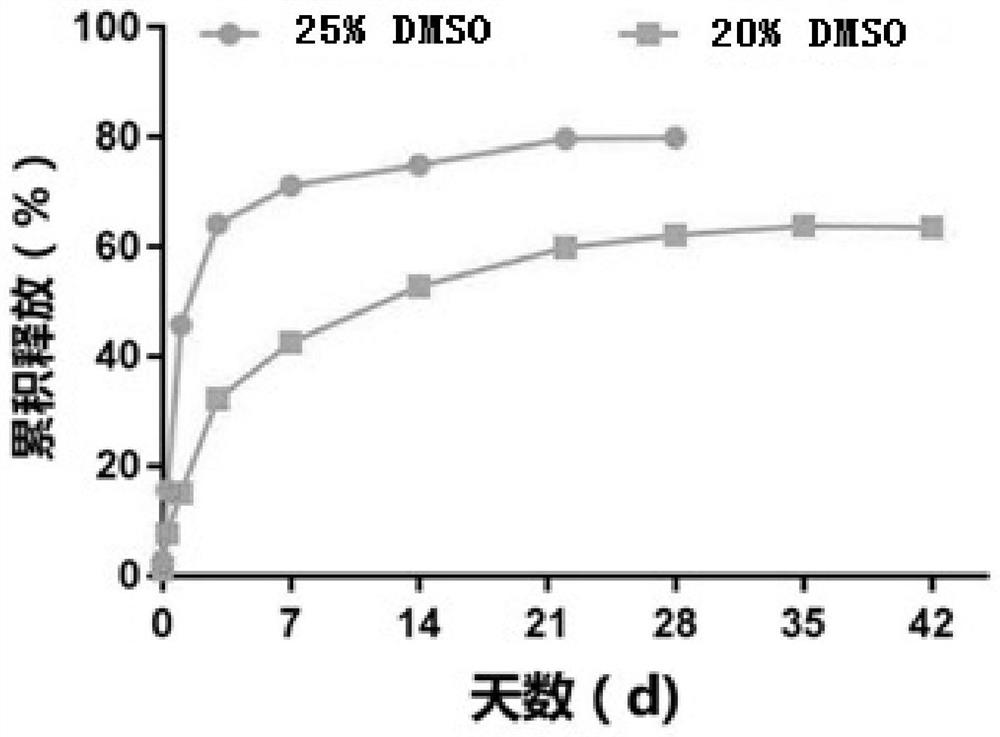
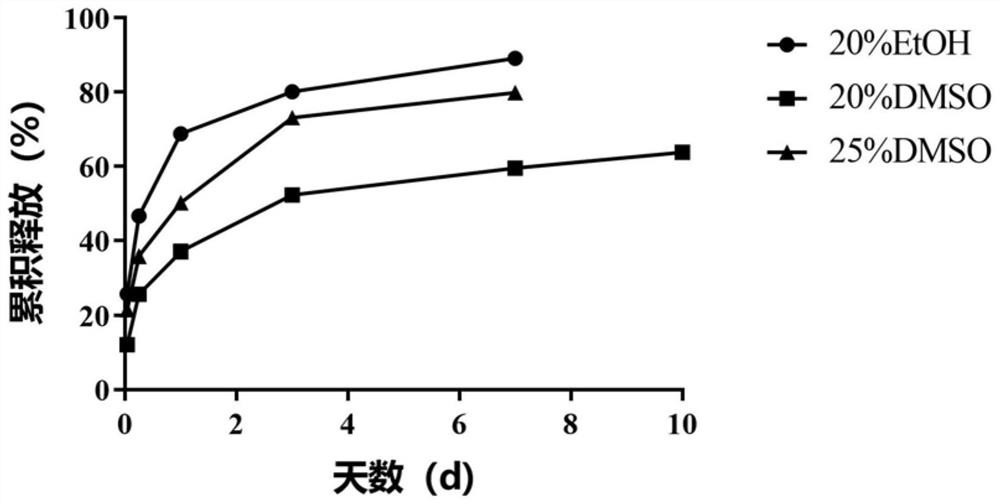
[0123] In this Example 2, the depot composition based on the liquid crystal drug delivery system according to the present invention was exemplarily prepared, and octreotide hydrochloride was used as a model peptide drug, showing the amount of organic solvent in the depot composition, different The types of organic solvents and the effects of different liquid crystal forming agents on the sustained release performance of liquid crystal drug delivery systems.
[0124] 2.1 The impact of the amount of organic solvent DMSO and different organic solvent types (DMSO or EtOH) on the sustained release performance of the liquid crystal drug delivery system in the storage composition:
[0125] 2.1.1 Prepare a peptide sustained-release preparation containing octreotide hydrochloride and SPC / SMO (Span 80) / DMSO depot composition, and its preparation method is as follows:
[0126] According to the...
Embodiment 3
[0163] Example 3: Preparation of peptide sustained-release preparations for injection according to the present invention (hereinafter referred to as "sustained-release preparations")
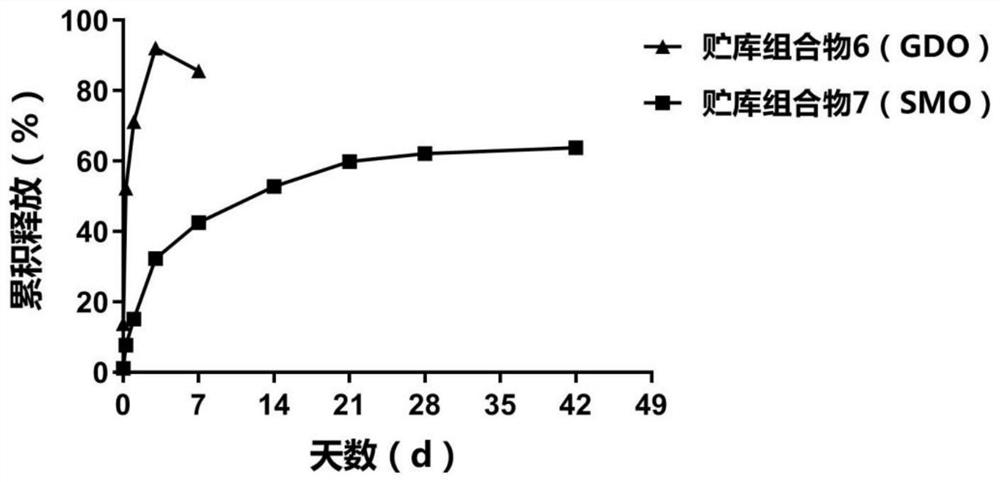
[0164] 3.1 Taking the pharmaceutical composition obtained according to the preparation method described in Example 1 as an example, combined with liquid crystal drug delivery system depot composition (SPC / SMO / DMSO, SPC / GMO / DMSO, or SPC / GDO / EtOH, Wherein, SMO is Span 80), an exemplary preparation of the peptide sustained-release preparation according to the present invention:
[0165] 3.1.1 Preparation of sustained-release formulation Control group A: containing octreotide acetate (OCT) and SPC / SMO / DMSO depot composition, its preparation method is as follows:
[0166] Add 37.5wt% SPC, 37.5wt% SMO, and 20wt% DMSO to the vial in sequence, and then incubate in a 60°C biochemical incubator to a uniform solution, then add 5% OCT, and mechanically stir with an anchor paddle to make it fully Mix to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com