Preparation method of 5-amino-1, 2, 3-benzene tricarboxylic acid
A technology of benzenetricarboxylic acid and amino, which is applied in the field of fine chemicals, can solve problems such as potential safety hazards, material volume, reaction temperature, and pressure that are difficult to control, and achieve the effects of improved safety, less liquid holding capacity, and uniform heat transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
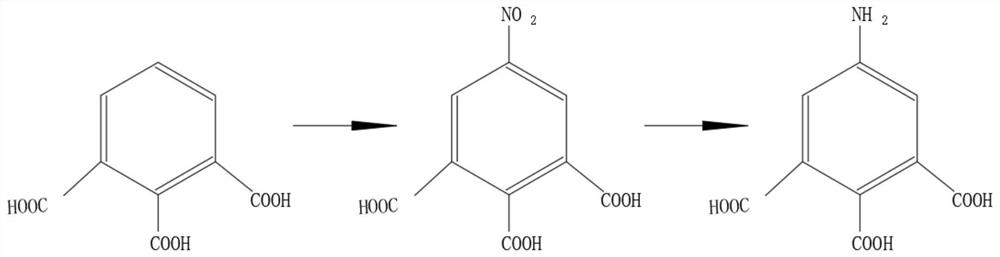
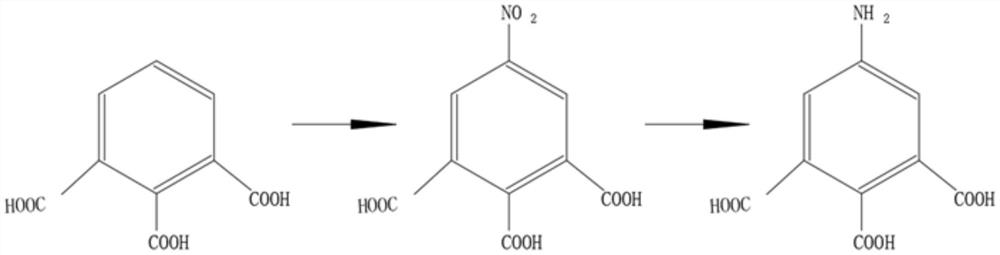
[0027] see figure 1 , the present invention provides the following technical solutions: a preparation method of 5-amino-1,2,3-benzenetricarboxylic acid, comprising the steps of:
[0028] Step 1: Dissolving 1,2,3-benzenetricarboxylic acid in nitric acid, wherein the molar ratio of 1,2,3-benzenetricarboxylic acid and nitric acid is 1:1-5, and the mass fraction of nitric acid is 60%-98% ;
[0029] Step 2: Put the nitric acid solution and sulfuric acid containing 1,2,3-benzenetricarboxylic acid into the microchannel reactor through a metering pump to react, the reaction temperature is 50°C-200°C, and the mass fraction of sulfuric acid is 80-98% or is oleum;
[0030] Step 3: Under the specified temperature conditions, after the reaction is completed, the reaction liquid obtained from the outlet of the microchannel reactor flows directly into the collector, and the temperature of the collector is kept at 0°C-5°C. After the reaction liquid stops flowing out, it is allowed to stand ...
Embodiment 1
[0040] The molar ratio of 1,2,3-benzenetricarboxylic acid to nitric acid is 1:1, and the mass fraction of nitric acid is 60%; the molar ratio of 1,2,3-benzenetricarboxylic acid, nitric acid, and sulfuric acid is 1:1:2; The mass ratio of palladium carbon catalyst, 5-nitro-1,2,3-benzenetricarboxylic acid and methanol is 0.01:1:10.
Embodiment 2
[0042] The molar ratio of 1,2,3-benzenetricarboxylic acid to nitric acid is 1:3, and the mass fraction of nitric acid is 79%; the molar ratio of 1,2,3-benzenetricarboxylic acid, nitric acid, and sulfuric acid is 1:3:4.5; The mass ratio of palladium carbon catalyst, 5-nitro-1,2,3-benzenetricarboxylic acid and methanol is 0.08:1:12.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com