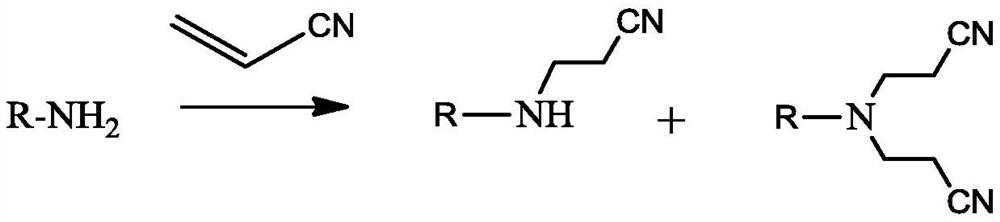
Method for synthesizing dinitrile ethyl tertiary amine from aliphatic primary amine by one-step method
A technology of aliphatic primary amines and tertiary amines, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitriles, etc., can solve problems such as increasing the complexity of the reaction process, complex reaction process, and large amount of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] reaction process:
[0039] Add 132.5g acrylonitrile and 2.65g glycolic acid aqueous solution (glycolic acid concentration is 70wt%, with the total weight of aqueous solution) in the 500ml three-neck flask that magneton is housed, adopt constant pressure dropping funnel to 73g n-butylamine in 30min Add it dropwise to a three-necked flask, and stir at reflux for 6h at 90°C.
[0040] Post-processing process:
[0041] The above reaction mother liquor was kept at 110°C and an absolute pressure of 5KPa, and a vacuum pump was used to remove light components such as acrylonitrile for about 3 hours, and the product liquid was analyzed by gas chromatography. , the mononitrile ethyl substituted n-butylamine content is 0.8wt%, and the n-butylamine content is 0.7wt%.
Embodiment 2
[0043] reaction process:
[0044] Add 116.6g acrylonitrile and 5.83g glycolic acid aqueous solution (glycolic acid concentration is 60wt%, with aqueous solution gross weight) in the 500ml three-neck flask that magneton is housed, adopt constant pressure dropping funnel to 99g cyclohexylamine in 40min Add it dropwise to a three-necked flask, and stir at reflux for 5h at 80°C.
[0045] Post-processing process:
[0046] The above reaction mother liquid was removed at 120°C and an absolute pressure of 10KPa, and light components such as acrylonitrile were removed by a vacuum pump for about 2 hours. The product liquid was analyzed by gas chromatography, and the content of dinitrile ethyl substituted cyclohexylamine was 98.8wt%. , the content of mononitrile ethyl substituted cyclohexylamine is 0.8wt%, and the content of cyclohexylamine is 0.4wt%.
Embodiment 3
[0048] reaction process:
[0049]Add 127.2g acrylonitrile and 6.36g glycolic acid aqueous solution (glycolic acid concentration is 70wt%, with the total weight of aqueous solution) in the 500ml three-necked flask that magneton is housed, adopt constant pressure dropping funnel to 113g 2-methylcyclohexane The amine was added dropwise to the three-necked flask within 60 minutes, and stirred at reflux at 90°C for 5 hours.
[0050] Post-processing process:
[0051] The above reaction mother liquid is at 120°C and the absolute pressure is 5KPa, and the light components such as acrylonitrile are removed by vacuum pump for about 3 hours, and the product liquid is analyzed by gas chromatography. It can be seen that the content of dinitrile ethyl substituted methylcyclohexylamine is 98.1 wt%, the content of mononitrile ethyl substituted methylcyclohexylamine is 1.2wt%, and the content of methylcyclohexylamine is 0.7wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com