Method for wet recovery of lithium elements from scrapped lithium iron phosphate batteries
A lithium iron phosphate battery, lithium iron phosphate technology, applied in battery recycling, waste collector recycling, non-metallic elements and other directions, can solve the problems of complex process, low lithium recovery rate and high recovery cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
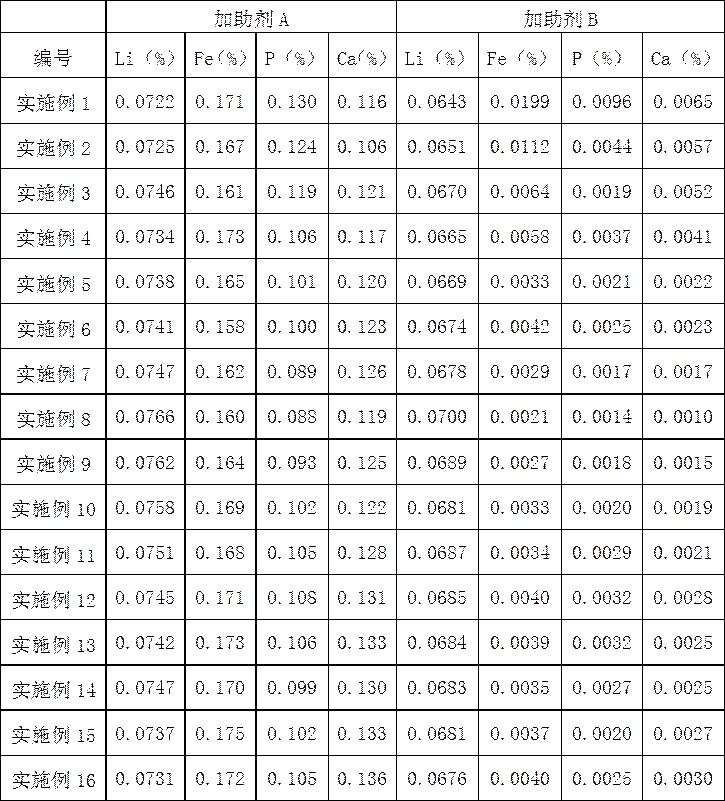
[0023] 1) Take ethylene carbonate (CE) 10%, bentonite 1%, calcium hydroxide (Ca(OH) 2 ) 87.7%, ethylenediaminetetraacetic acid (EDTA) 1%, dispersant CNF0.1% (purchased from Guangzhou Lihongji Chemical Co., Ltd.), AGITAN P803 0.1% (purchased from Nanjing Yuelai New Material Technology Co., Ltd.), fatty acid Polyoxyethylene ester 0.1%, mix the raw materials taken evenly, and ball mill at 250r / min for 1h to obtain 20g of additive A;
[0024] 2) Take ethylene carbonate (CE) 0.1%, ethylenediaminetetraacetic acid (EDTA) 1%, sodium hydroxide (NaOH) 97.6%, ammonium bicarbonate (NH 4 HCO 3 ) 1%, polyacrylamide ((C 3 h 5 NO)n) 0.1%, sodium dodecylbenzenesulfonate 0.1%, organopolysiloxane 0.1%, mix the raw materials evenly, and ball mill at 250r / min for 1h to obtain 20g of additive B;
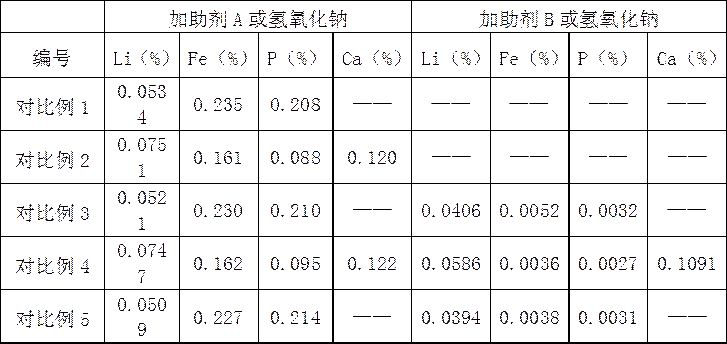
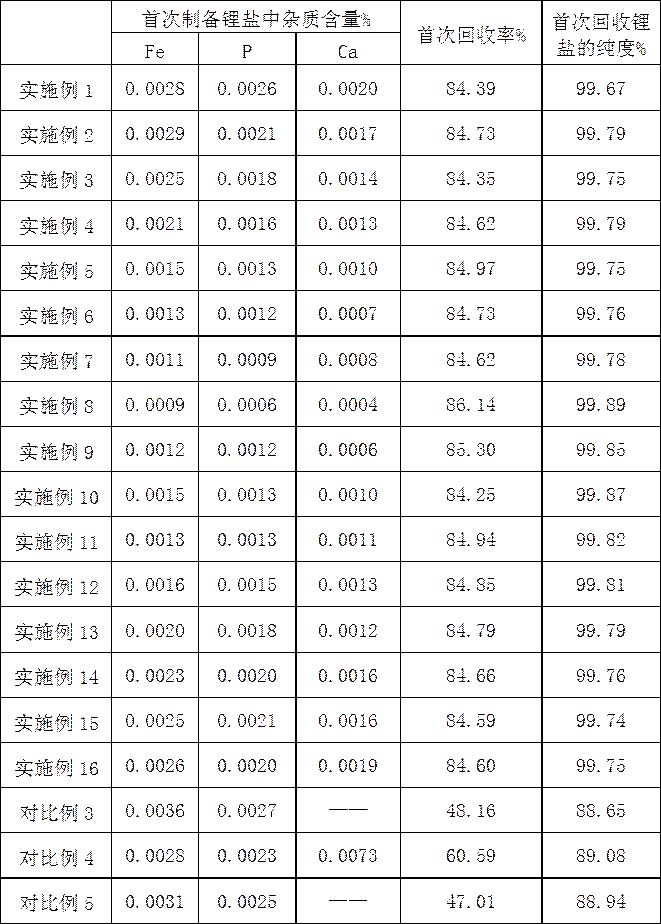
[0025] 3) Take 15.7760g of lithium iron phosphate positive electrode active material and use sulfuric acid with a mass concentration of 2% for acid leaching. The molar mass ratio of lithium iron phosp...
Embodiment 2
[0031] 1) Take ethylene carbonate (CE) 10%, bentonite 1%, calcium hydroxide (Ca(OH) 2 ) 87.7%, ethylenediaminetetraacetic acid (EDTA) 1%, dispersant CNF0.1%, AGITAN P803 0.1%, fatty acid polyoxyethylene ester 0.1%, the raw materials were mixed evenly, 300r / min ball milled for 1h, and the auxiliary Agent A20g;
[0032] 2) Take ethylene carbonate (CE) 0.1%, ethylenediaminetetraacetic acid (EDTA) 1%, sodium hydroxide (NaOH) 97.6%, ammonium bicarbonate (NH 4 HCO 3 ) 1%, polyacrylamide ((C 3 h 5 NO)n) 0.1%, sodium dodecylbenzenesulfonate 0.1%, organopolysiloxane 0.1%, mix the raw materials evenly, and ball mill at 300r / min for 1h to obtain additive B20g
[0033] 3) Take 15.7760g of lithium iron phosphate positive electrode active material and use sulfuric acid with a mass concentration of 2% for acid leaching. The molar mass ratio of lithium iron phosphate to sulfuric acid is 1:2, the reaction time is 1h, the reaction temperature is 45°C, and the reaction ends After filtering,...
Embodiment 3
[0039] 1) Take ethylene carbonate (CE) 10%, bentonite 1%, calcium hydroxide (Ca(OH) 2 ) 87.7%, ethylenediaminetetraacetic acid (EDTA) 1%, dispersant CNF0.1%, AGITAN P803 0.1%, fatty acid polyoxyethylene ester 0.1%, the raw materials were mixed evenly, 350r / min ball milled for 1h, and the auxiliary Agent A20g;
[0040] 2) Take ethylene carbonate (CE) 0.1%, ethylenediaminetetraacetic acid (EDTA) 1%, sodium hydroxide (NaOH) 97.6%, ammonium bicarbonate (NH 4 HCO 3 ) 1%, polyacrylamide ((C 3 h 5 NO)n) 0.1%, sodium dodecylbenzenesulfonate 0.1%, organopolysiloxane 0.1%, mix the raw materials evenly, and ball mill at 350r / min for 1h to obtain 20g of additive B;
[0041] 3) Take 15.7760g of lithium iron phosphate positive electrode active material and use sulfuric acid with a mass concentration of 2% for acid leaching. The molar mass ratio of lithium iron phosphate to sulfuric acid is 1:2, the reaction time is 1h, the reaction temperature is 45°C, and the reaction ends After filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com